Phage-like particles tests
Phage-like particles (PLPs) in the soil
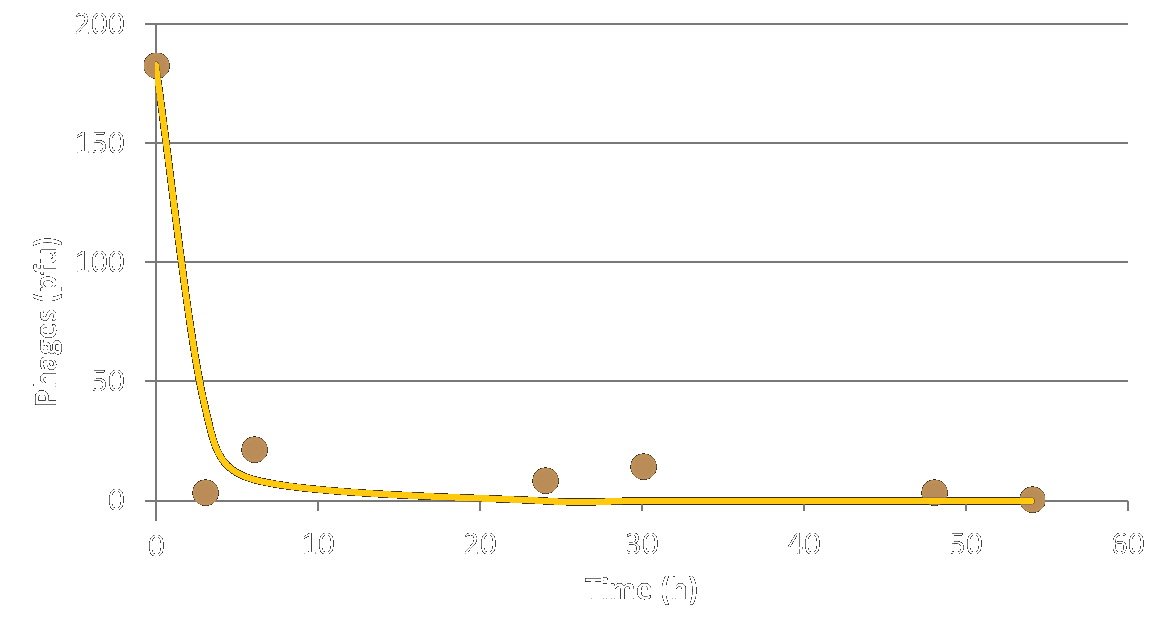
We first tested the lifetime of our phage-like particles (PLPs) in the soil. The soil samples can from the Moulin de Velaux which is an olive grower cooperative in southern France. For this experiment, we put M13 phages in the soil and we let it incubate for different amounts of time. As seen in figure 1, the population of phage decreases with time because they are degraded by micro-organisms that lived in the soil. The experimental half-life of our phage-like particles is approximately of 4 hours.
With this result, we can be confident that our phage-like particles solution doesn't pollute the soil. This test is one of the many tests needed for the marketing authorization procedure of our production. Check out our legislation page for more information.
Test with Arabidopsis thaliana
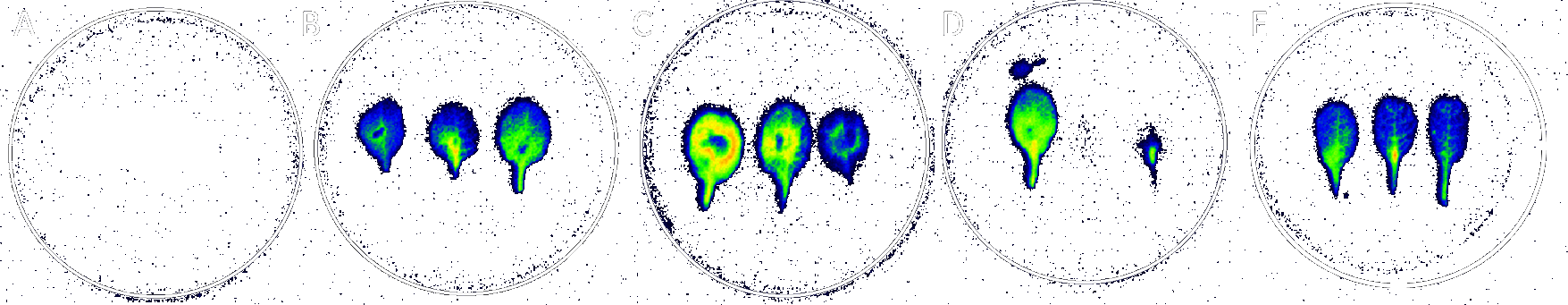
The farmer's surveys indicated that for user acceptability, the application method for our treatment was important. In order to find possible ways of applying the treatment to a tree we tested multiple ways with our model plant Arabidopsis thaliana. To observe the movement of PLPs in plants, we attached a fluorochrome to our phage-like particles (PLPs), making a solution of fluorescent PLPs. Then, this solution was used on Arabidopsis thaliana in different ways :
- pricking the leaf (which mimics the injection).
- infiltrating the solution into the leaf.
- putting a drop of solution on the leaf (to mimic a spray).
- soaking the petiole of a leaf in the solution.
The control is leaf without any fluorescent PLPs. As you can see the use of pricking, infiltration and soaking the petiole are all more efficient than using a drop.
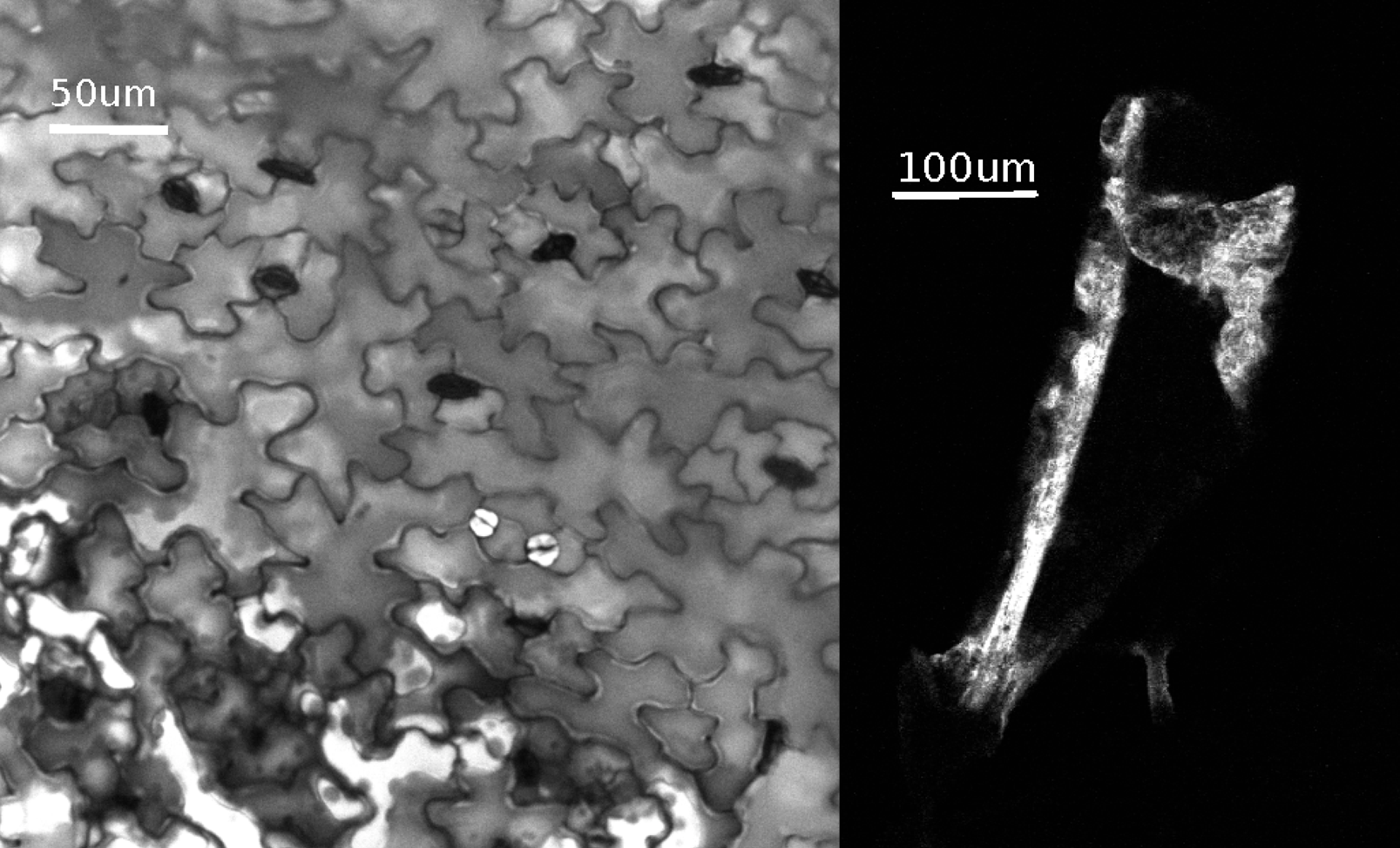
Finally, we wanted to know if the phage was able to circulate to different parts of the leaf. We can see that all the cells become fluorescent. Thus, PLPs are not confined to leaf vessels but can circulate freely in the plant.
PLPs rate of production
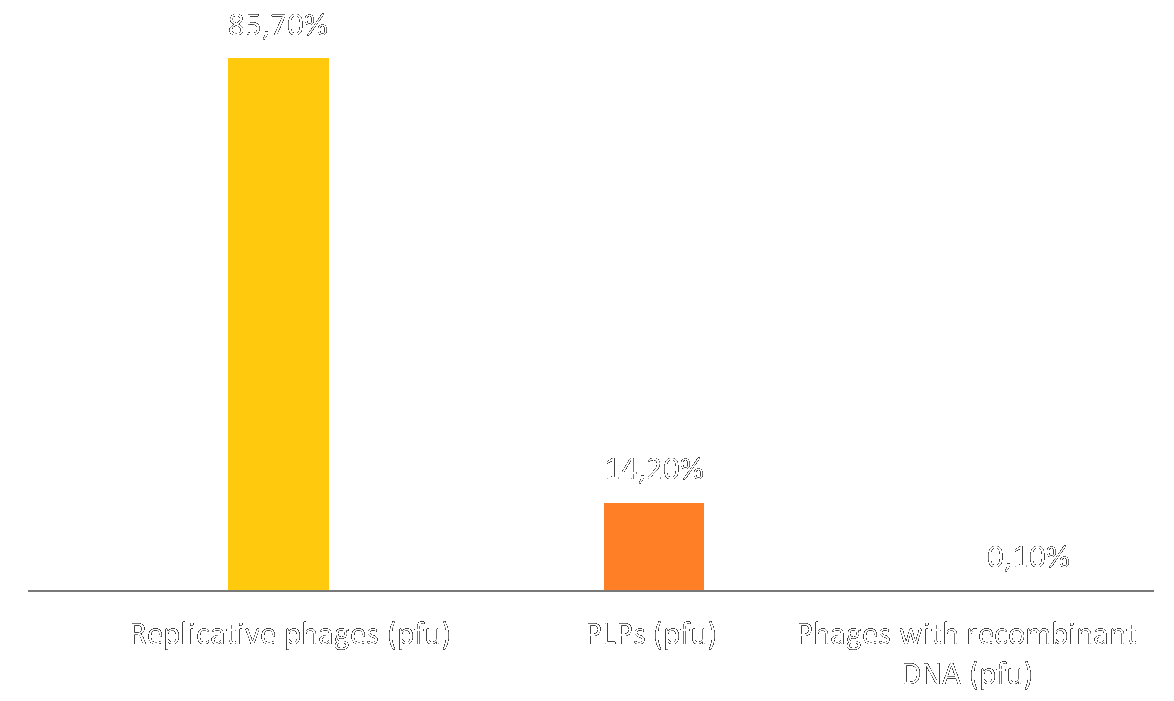
Thanks to the interview with Jacques VAN HELDEN we considered the production rate of our PLPs and the risk of recombination between M13KO7 and the phagemid. This question matters because we aim to create PLPs that are unable to replicate and thus spread in the environment. Therefore, we tested this possibility in the lab and also with our model.
As you can see, recombination is very rare and only occurs for 0,1% of phages, this is coherent with our assumptions. However unfortunately in these tests, PLPs represent only 14,2% of the production against 85,7% for the replicative phage. This means that the vast majority of our production is phages containing M13KO7 DNA and that they cannot inject toxins.