Zsy5691750 (Talk | contribs) |
|||
| Line 437: | Line 437: | ||
<h5>2) Verification of RFP in the TVRVC</h5> | <h5>2) Verification of RFP in the TVRVC</h5> | ||
<hr> | <hr> | ||
| − | <p>The main | + | <p>The main characterization method of verification of <i>RFP</i> in the TVRVC applied by us is observing the expression of <i>red fluorescent protein</i> under the fluorescence microscope. By this way, it will be much more intuitive so that we can directly get the results. We took pictures under different visions and the results are as follows.All the experiment including PVRVC regulation system use this assay method.</p> |
<div id="threepic1"> | <div id="threepic1"> | ||
| Line 513: | Line 513: | ||
<p>There are 325 yellow colonies and 31 white colonies in the field of view.</p> | <p>There are 325 yellow colonies and 31 white colonies in the field of view.</p> | ||
<p>Apart from mating, we also transformed plasmid <i>pRS416</i> with <i>vika</i> gene into the PVRVC. The efficiency is up to 91.3 percent in this figure.</p> | <p>Apart from mating, we also transformed plasmid <i>pRS416</i> with <i>vika</i> gene into the PVRVC. The efficiency is up to 91.3 percent in this figure.</p> | ||
| − | <p>Compare above two methods, mating and transformation of plasmid, we find that mating is not as efficient as the transformation of the plasmid. After analysis, we came to the conclusions as follows. For the mating method, <i>vika recombinase</i> has stop expressing when <i>BY4742</i> mated with PVRVC in YPD medium. The previously expressed | + | <p>Compare above two methods, mating and transformation of plasmid, we find that mating is not as efficient as the transformation of the plasmid. After analysis, we came to the conclusions as follows. For the mating method, <i>vika recombinase</i> has stop expressing when <i>BY4742</i> mated with PVRVC in YPD medium. The previously expressed Vika recombinase may be degraded during the growth. In contrast to this, with another method that the plasmid was transformed into PVRVC directly, <i>vika recombinase</i> is continuously expressed during cell growth. So the efficiency of the second method is higher than the first method.</p> |
<div class="zxx_zoom_demo_qqqqq" align="center"> | <div class="zxx_zoom_demo_qqqqq" align="center"> | ||
| Line 542: | Line 542: | ||
<p>These are parts of a successful result of mating mentioned above. Two figures one by one correspondence.</p> | <p>These are parts of a successful result of mating mentioned above. Two figures one by one correspondence.</p> | ||
| − | <p>To sum up, mating switcher can be presented in kinds of yeast with different forms. This proves that our Mating switcher is fast, flexible and efficient.</p> | + | <p>To sum up, the mating switcher can be presented in kinds of yeast with different forms. This proves that our Mating switcher is fast, flexible and efficient.</p> |
<p>Meantime, we cultured the transformed yeast in several 5mL liquid <i>SC-Leu</i> at 30℃ and 220 rpm for 12 hours ( Take three samples at a time). We used one sample for centrifugation to precipitate the bacterial and the remaining two remained unchanged. The difference is the fluorescence value we need, then we calculated the value of average them. The excitation wavelength is set at 540nm and the emission wavelength is set at 635nm. Hereafters, we measured the yeast concentration at OD<sub>600</sub>. At last, we divided the fluorescence value by OD<sub>600</sub> to normalize the value and the result data is as follows. | <p>Meantime, we cultured the transformed yeast in several 5mL liquid <i>SC-Leu</i> at 30℃ and 220 rpm for 12 hours ( Take three samples at a time). We used one sample for centrifugation to precipitate the bacterial and the remaining two remained unchanged. The difference is the fluorescence value we need, then we calculated the value of average them. The excitation wavelength is set at 540nm and the emission wavelength is set at 635nm. Hereafters, we measured the yeast concentration at OD<sub>600</sub>. At last, we divided the fluorescence value by OD<sub>600</sub> to normalize the value and the result data is as follows. | ||
</p> | </p> | ||
| Line 564: | Line 564: | ||
<p>Our mating switch plays an important role in many respects, such as including heavy metal treatment and cell signal switching. And we created a novel method to prove the effectiveness of the switch in an intuitive and effective way. The terminator of the first part (PVUVC) terminates the expression of the downstream gene, proving the validity of the switcher, and the second part (PVRVC) creates an evident method of color conversion to determine the state of the switcher.</p> | <p>Our mating switch plays an important role in many respects, such as including heavy metal treatment and cell signal switching. And we created a novel method to prove the effectiveness of the switch in an intuitive and effective way. The terminator of the first part (PVUVC) terminates the expression of the downstream gene, proving the validity of the switcher, and the second part (PVRVC) creates an evident method of color conversion to determine the state of the switcher.</p> | ||
| − | <p>Aiming to increase the | + | <p>Aiming to increase the Vika-vox system efficiency, we let Vika enzyme saturate expression, but the efficiency was still relatively low. We hypothesized that this phenomenon was caused by degradation of the Vika enzyme in the YPD culture medium. We’d better change the composition or proportion of YPD ingredients to find out the best culture conditions. We are looking forward to more research in this field so that we can make this system work better and even perfectly.</p> |
<p>We use the <i>RFP</i> as the reporting protein. But there exists a drawback that it’s detected with an expensive device. A more intuitive reporting strategy need to be developed, maybe it can be seen by bare eyes like <i>E.coli</i> in the near future.</p> | <p>We use the <i>RFP</i> as the reporting protein. But there exists a drawback that it’s detected with an expensive device. A more intuitive reporting strategy need to be developed, maybe it can be seen by bare eyes like <i>E.coli</i> in the near future.</p> | ||
| Line 822: | Line 822: | ||
<hr> | <hr> | ||
| − | <p>The Cu-induced promoter <i>CUP1</i> promoter is a previous BioBrick used by iGEM16_Washington, iGEM16_Waterloo, and other iGEM teams. However, the detailed characterization like what we did this year hasn't been shown on iGEM parts page. Moreover, this part hasn’t be improved by any means or in any | + | <p>The Cu-induced promoter <i>CUP1</i> promoter is a previous BioBrick used by iGEM16_Washington, iGEM16_Waterloo, and other iGEM teams. However, the detailed characterization like what we did this year hasn't been shown on iGEM parts page. Moreover, this part hasn’t be improved by any means or in any way. Under this situation, we plan to work on this promoter to improve its sensitivity and response peak, reduce the leakage expression, and create new parts for future work.</p> |
| Line 857: | Line 857: | ||
</div> | </div> | ||
| − | <p>Based on this mechanism, we redesigned the part sequence provided by iGEM16_Washington. We deleted irrelevant | + | <p>Based on this mechanism, we redesigned the part sequence provided by iGEM16_Washington. We deleted irrelevant based on the two ends of this promoter and retained the core sequence. In this way, this promoter played its key role with fewer bases. Strains of <i>S. cerevisiae BY4742</i> containing either BBa_K2165004-yEmRFP and BBa_K2407000-yEmRFP with an initial OD<sub>600</sub> of 0.1 were grown for 24 hours in SC-URA medium at 30 degrees Celsius, and then were induced with 0.1 mM Cu<sup>2+</sup>. Samples were tested with fluorescent spectrophotometer (Hitachi F-2700) after 1, 3, 6, 12, and 24 hours.</p> |
<div class="row"> | <div class="row"> | ||
| Line 989: | Line 989: | ||
<p>The figure shows the response ranges of biosensors with different promoters within 20 min. For most biosensors, the fluorescence intensity increases as copper ion’s concentration increases from 0 to 100 μM. However, when the concentration exceeds 100 μM, the responses of most biosensor become slow, and the fluorescence intensity decreases. A reasonable explanation is that high concentrations of copper can inhibit the biosensor's response within a short time. </p> | <p>The figure shows the response ranges of biosensors with different promoters within 20 min. For most biosensors, the fluorescence intensity increases as copper ion’s concentration increases from 0 to 100 μM. However, when the concentration exceeds 100 μM, the responses of most biosensor become slow, and the fluorescence intensity decreases. A reasonable explanation is that high concentrations of copper can inhibit the biosensor's response within a short time. </p> | ||
| − | <p>Fortunately, we still found a biosensor who met the requirements of an excellent biosensor. EP-5 has a less leakage and a higher sensitivity. Its fluorescence intensity is lower than the control group by 17 units with no induction and is higher by 21 units with 100-μM-Cu induction. By aligning the sequence with the CUP1 promoter, we found altered bases mainly located at | + | <p>Fortunately, we still found a biosensor who met the requirements of an excellent biosensor. EP-5 has a less leakage and a higher sensitivity. Its fluorescence intensity is lower than the control group by 17 units with no induction and is higher by 21 units with 100-μM-Cu induction. By aligning the sequence with the CUP1 promoter, we found altered bases mainly located at both sides of UASs and a deletion of one base even occurred between two UASs. We suspected that the change of sensitivity and leakage expression mainly due to the change of spatial distribution and the increase of A/T concentration, which both could influence the binding procedure of transcription factors.</p> |
<div class="row"> | <div class="row"> | ||
| Line 1,023: | Line 1,023: | ||
<hr> | <hr> | ||
| − | <p>In our characterization of both primary and improved promoters, we found the effect of induction is not as obvious as expected (Previous iGEM team’s results). After reading some references, we found the activation process is related to the acetylation of H3 and H4 located at <i>CUP1</i> promoter, which showed nucleosome reposition and transcription factors binding might be the main reason for the activation. However, our biosensors were ligated on plasmid pRS416, which usually exists in the nucleus in a supercoiled state. There is only little possibility for a plasmid to binds to histones, so the transcription process shows less activation than that on a chromosome.</p> | + | <p>In our characterization of both primary and improved promoters, we found the effect of induction is not as obvious as expected (Previous iGEM team’s results). After reading some references, we found the activation process is related to the acetylation of H3 and H4 located at the <i>CUP1</i> promoter, which showed nucleosome reposition and transcription factors binding might be the main reason for the activation. However, our biosensors were ligated on plasmid pRS416, which usually exists in the nucleus in a supercoiled state. There is only little possibility for a plasmid to binds to histones, so the transcription process shows less activation than that on a chromosome.</p> |
<p>In the future, we plan to construct this biosensor on chromosomes to see whether the result will be more positive. Meanwhile, we will continue enlarging the response peak and range to improve this biosensor.</p> | <p>In the future, we plan to construct this biosensor on chromosomes to see whether the result will be more positive. Meanwhile, we will continue enlarging the response peak and range to improve this biosensor.</p> | ||
| Line 1,084: | Line 1,084: | ||
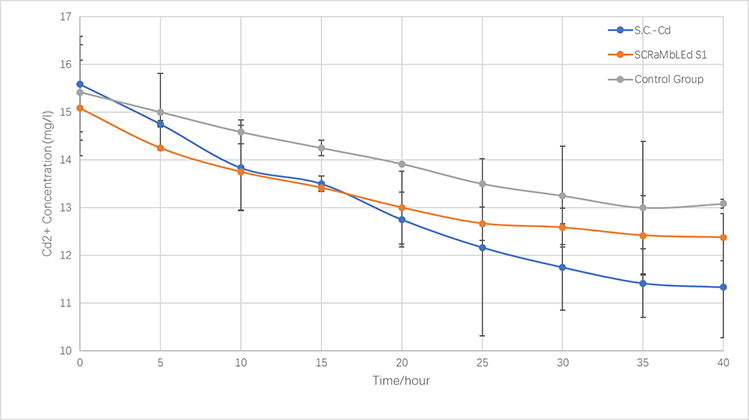
<p>In terms of the respective ability to adsorb copper and cadmium, we compare genetically-engineered yeast, SCRaMbLE yeast and original one. | <p>In terms of the respective ability to adsorb copper and cadmium, we compare genetically-engineered yeast, SCRaMbLE yeast and original one. | ||
| − | <p>As is illustrated in Fig.5-1 and Fig.5-2, engineered yeast significantly absorbs more ion than control group without any improvement. Furthermore, SCRaMbLE yeast also shows excellent adsorption capacity, comparable to genetically-engineered one. Fig.X1 reveals the | + | <p>As is illustrated in Fig.5-1 and Fig.5-2, engineered yeast significantly absorbs more ion than the control group without any improvement. Furthermore, SCRaMbLE yeast also shows excellent adsorption capacity, comparable to genetically-engineered one. Fig.X1 reveals the adsorption of copper ion, which relatively faster than cadmium, showed in Fig.X2.</p> |
<div class="zxx_zoom_demo_qqqqqqq"> | <div class="zxx_zoom_demo_qqqqqqq"> | ||
<div class="small_pic_demo_qqqqqqq" style="float:left;"> | <div class="small_pic_demo_qqqqqqq" style="float:left;"> | ||
Revision as of 11:50, 31 October 2017
/* OVERRIDE IGEM SETTINGS */