Liu Zichen (Talk | contribs) |
|||
| Line 217: | Line 217: | ||
<h2><i class="fa fa-university" aria-hidden="true"></i> Background</h2> | <h2><i class="fa fa-university" aria-hidden="true"></i> Background</h2> | ||
| − | <p>Human existence on earth is almost impossible without the heavy metals. Even though important to mankind, exposure to them during production, usage and their uncontrolled discharge | + | <p>Human existence on earth is almost impossible without the heavy metals. Even though important to mankind, exposure to them during production, usage and their uncontrolled discharge into the environment has caused lots of hazards to man, other organisms and the environment itself. Heavy metals can enter human tissues and organs via inhalation, diet, and manual handling. As the process of urbanization and industrialization goes deeper and deeper, heavy metal pollution, a noticeable threaten to almost all the creatures, has become an essential problem to solve.</p> |
| − | <p>According to our human practice, the situation of heavy metal pollution (copper and cadmium ions) is marked on a world map, and the severity of heavy metal pollution has been increasing all over this map. Places with serious pollution | + | <p>According to our human practice, the situation of heavy metal pollution (copper and cadmium ions) is marked on a world map, and the severity of heavy metal pollution has been increasing all over this map. Places with serious pollution include middle Asia, eastern Asia, southern Europe, and Latin America. In addition, not only fresh water source, but also soil and crops are seriously contaminated by heavy metals. On average, during three out of ten suppers we have, we absorb excess heavy metals over the standard concentration.</p> |
<p>Considering the rigorous situation we face, our team decided to design an advanced system for typical toxic heavy metal disposal based on Saccharomyces cerevisiae.</p> | <p>Considering the rigorous situation we face, our team decided to design an advanced system for typical toxic heavy metal disposal based on Saccharomyces cerevisiae.</p> | ||
</div> | </div> | ||
| Line 238: | Line 238: | ||
<hr> | <hr> | ||
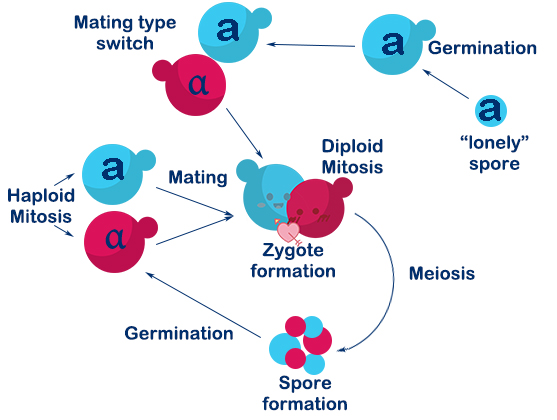
<p>After we found there might be revolutionary usage about the mating type switch (MTS) of yeasts in our heavy metal deposition system, In laboratory, the species of budding yeast we usually used are BY4741 and BY4742, whose HO gene are knocked out. Therefore, we intended to use two groups of MATa yeasts to realize the mating switcher. </p> | <p>After we found there might be revolutionary usage about the mating type switch (MTS) of yeasts in our heavy metal deposition system, In laboratory, the species of budding yeast we usually used are BY4741 and BY4742, whose HO gene are knocked out. Therefore, we intended to use two groups of MATa yeasts to realize the mating switcher. </p> | ||
| − | <p>One of these groups was required to achieve MTS. We decided to achieve MTS by introducing the HO gene into this group of yeasts— Saccharomyces cerevisiae (BY4741, in our lab, whose chromosome Ⅹhas been switched by synthetic chromosome Ⅹ. And it has been renamed as SynⅩsimilarly hereinafter | + | <p>One of these groups was required to achieve MTS. We decided to achieve MTS by introducing the HO gene into this group of yeasts— Saccharomyces cerevisiae (BY4741, in our lab, whose chromosome Ⅹhas been switched by synthetic chromosome Ⅹ. And it has been renamed as SynⅩsimilarly hereinafter). To make the MTS controllable, it is necessary for us to adopt inducible promoters to initiate the expression of HO gene or create a pathway functioning as single gene regulator. Eventually, we landed on the Gal1 promoter first, for its convenience and efficiency. As we read in R. Scott McIsaac’s work, their rapid, tunable, single-gene specificity control system of single gene in yeasts has given us much impression. Therefore, we decided to use this system as one of our pathway designs for the expression of HO gene. </p> |
<h4>GETTING THE CHASSIS </h4> | <h4>GETTING THE CHASSIS </h4> | ||
<hr> | <hr> | ||
| − | <p>Aiming to achieve MTS for environmental use, it is essential to make sure that when the MAT locus has DSB(double strands break) cleaved by HO, our type-a (MATa) yeast can only become type-α (MATα). Therefore, we used a Ura-tag to replace the HMR(a) domain in chromosome Ⅲ. In this way the HMR will no longer be the donor for the homologous recombination in the repairing process for MAT cleavage. </p> | + | <p>Aiming to achieve MTS for environmental use, it is essential to make sure that when the MAT locus has DSB(double strands break) cleaved by HO, our type-a (MATa) yeast can only become type-α (MATα). Therefore, we used a Ura-tag to replace the HMR(a) domain in chromosome Ⅲ. In this way, the HMR will no longer be the donor for the homologous recombination in the repairing process for MAT cleavage. </p> |
| − | <p>Since the change of mating type may appear successively, there is a great possibility that the same type haploid mate with each other. To avoid the existence of meaningless mating , we built | + | <p>Since the change of mating type may appear successively, there is a great possibility that the same type haploid mate with each other. To avoid the existence of meaningless mating, we built a vector to express MATα genes to produce a1-α2 stable corepressor so that the haploid will regard itself as a diploid and prevent mating unless the MATa locus changes to the other one. After selection, by homologous recombination, we deleted the Ura-tag for further usage. We selected the target colonies(SynⅩdUra) via 5Foa plates. </p> |
<div class="zxx_zoom_demo_long" align="center"> | <div class="zxx_zoom_demo_long" align="center"> | ||
<script type="text/javascript" src="https://2017.igem.org/Team:Tianjin/Resources/JS:zoom?action=raw&ctype=text/javascript"></script> | <script type="text/javascript" src="https://2017.igem.org/Team:Tianjin/Resources/JS:zoom?action=raw&ctype=text/javascript"></script> | ||
| Line 259: | Line 259: | ||
<h5> 1) Gal System</h5> | <h5> 1) Gal System</h5> | ||
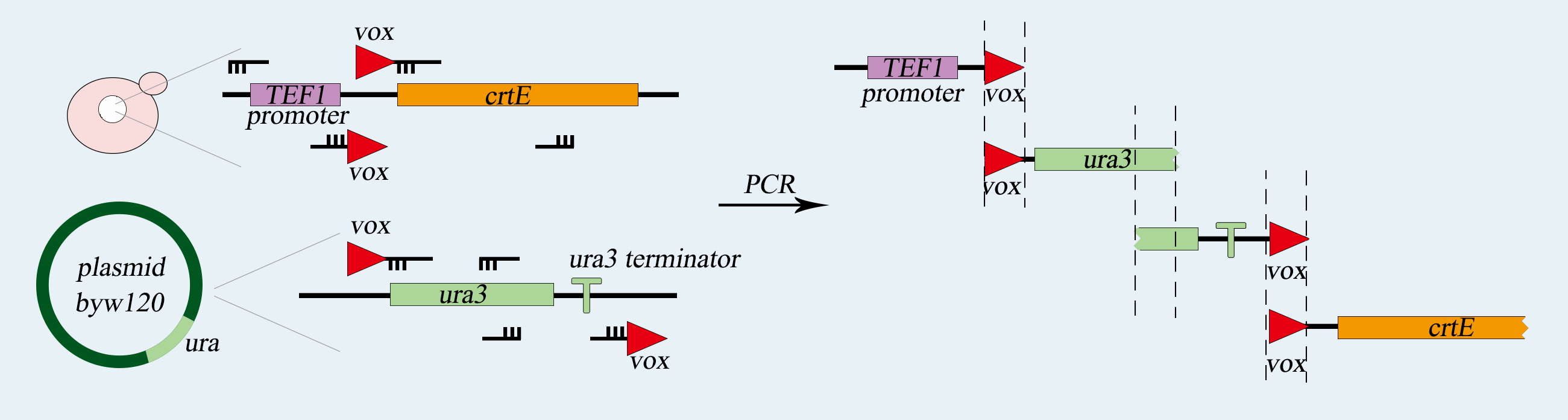
| − | <p>In this pathway, we chose Gal1 as our promoter for the expression of HO gene, CYC1 as the terminator, and PRS416(with Ura-tag) as our vector. As for segments ligation, we design the cutting sites for Bsa1 enzyme in each part, hoping to achieve seamless ligation of these three parts. </p> | + | <p>In this pathway, we chose Gal1 as our promoter for the expression of HO gene, CYC1 as the terminator, and PRS416(with Ura-tag) as our vector. As for segments ligation, we design the cutting sites for the Bsa1 enzyme in each part, hoping to achieve seamless ligation of these three parts. </p> |
<p>We adopted the PCR method to amplify the Gal1-part and CYC1-part from a Gal1-Vika plasmid we had used in our former lab work. We designed specific primers for this procedure. After PCR, the Gal1 has the cutting sites for SalⅠand BsaⅠon both ends, and CYC1 has that for BsaⅠand BamhⅠon both ends. Meanwhile, the HO gene was obtained by gene synthesis, flanked by specific hangtags for BsaⅠto be cohesive with Gal1 upstream and CYC1 downstream. Thus, we have built our composite part (GHC).</p> | <p>We adopted the PCR method to amplify the Gal1-part and CYC1-part from a Gal1-Vika plasmid we had used in our former lab work. We designed specific primers for this procedure. After PCR, the Gal1 has the cutting sites for SalⅠand BsaⅠon both ends, and CYC1 has that for BsaⅠand BamhⅠon both ends. Meanwhile, the HO gene was obtained by gene synthesis, flanked by specific hangtags for BsaⅠto be cohesive with Gal1 upstream and CYC1 downstream. Thus, we have built our composite part (GHC).</p> | ||
<p>After the ligation, we transformed the E.coli for the augment of the PRS416 plasmid with GHC. (GHC-416) And eventually, we transformed our SynⅩ-dUra for the GHC-416 to get our target yeasts——SynⅩ-dUra-416. </p> | <p>After the ligation, we transformed the E.coli for the augment of the PRS416 plasmid with GHC. (GHC-416) And eventually, we transformed our SynⅩ-dUra for the GHC-416 to get our target yeasts——SynⅩ-dUra-416. </p> | ||
| Line 265: | Line 265: | ||
<h5> 2) Modified. Gal1 system </h5> | <h5> 2) Modified. Gal1 system </h5> | ||
| − | <p>In this pathway, we introduced one kind of artificial transcription factor (ATF)—Z4EV into the | + | <p>In this pathway, we introduced one kind of artificial transcription factor (ATF)—Z4EV into the regulation of HO gene expression. With Z4EV working with our Modified. Gal1 promoter, we hoped to reach the on off-target dynamic control of HO gene expression. |
Our designing for getting the Modified. Gal1-HO-CYC1 parts (MGHC) is quite the same as that for GHC mentioned above, only that we acquired our Modified. Gal1 part from the gene synthesis. </p> | Our designing for getting the Modified. Gal1-HO-CYC1 parts (MGHC) is quite the same as that for GHC mentioned above, only that we acquired our Modified. Gal1 part from the gene synthesis. </p> | ||
<div class="zxx_zoom_demo_long" align="center"> | <div class="zxx_zoom_demo_long" align="center"> | ||
| Line 280: | Line 280: | ||
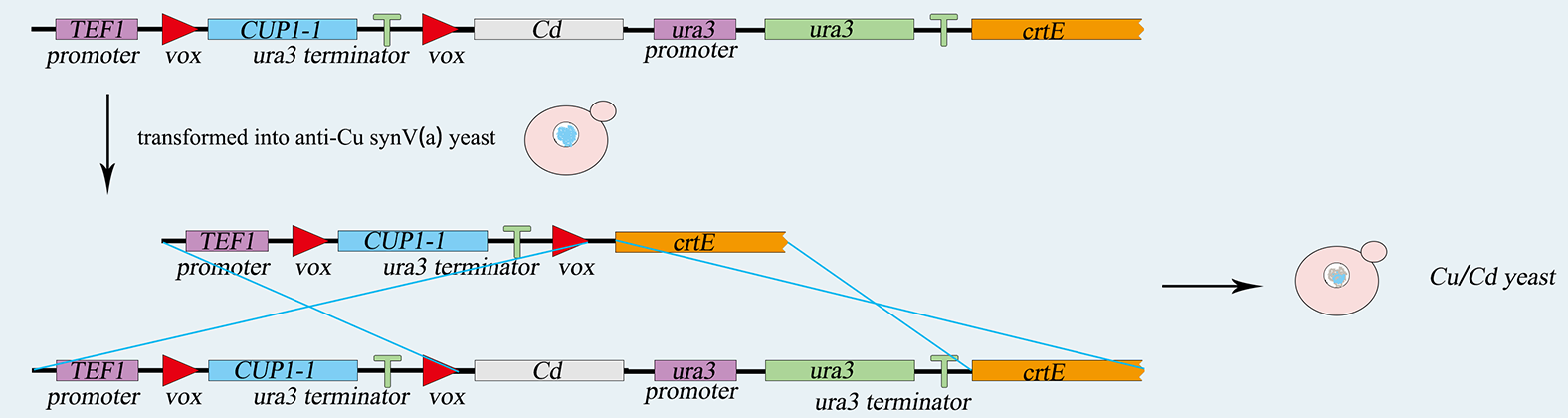
| − | <p>As for the expression of Z4EV gene, we intended to induce it into the SynX chromosome in SynⅩ-dUra by homologous recombination. With overlap PCR strategy, we put | + | <p>As for the expression of Z4EV gene, we intended to induce it into the SynX chromosome in SynⅩ-dUra by homologous recombination. With overlap PCR strategy, we put a homologous domain CanA (originally from Can gene in chromosome X) in the upstream of the promoter of Z4EV. Thus, we got our CanA-TEF-Z4EV part. Then we planned to use Tdh2t as terminator attached with Leu-CanB (another part of Can gene). </p> |
| − | <p>Next, for double using the Leu-tag, we introduce Vika/vox system. We intended to attach the Vika operator (Tdh3p- | + | <p>Next, for double using the Leu-tag, we introduce Vika/vox system. We intended to attach the Vika operator (Tdh3p-Vika-Tdh2t, TVT) following the Z4EV gene. We had two groups of yeasts, as mentioned above, one of them aimed to accomplish MTS and becoming MATα, the other with functional genes remained as MATa. According to our design, the former will express Vika recombinase, and the other contains functional genes whose expressions are controlled by vox-Terminator-vox structure. Thus, the function gene’s expression will be initiated during the cell fusion in yeast mating process. </p> |
<div class="zxx_zoom_mid" align="center"> | <div class="zxx_zoom_mid" align="center"> | ||
<script type="text/javascript" src="https://2017.igem.org/Team:Tianjin/Resources/JS:zoom?action=raw&ctype=text/javascript"></script> | <script type="text/javascript" src="https://2017.igem.org/Team:Tianjin/Resources/JS:zoom?action=raw&ctype=text/javascript"></script> | ||
| Line 297: | Line 297: | ||
<h4>TEST of MTS </h4> | <h4>TEST of MTS </h4> | ||
<hr> | <hr> | ||
| − | <p>In this section, we only got to test the Gal System due to time limits. And we figured that the | + | <p>In this section, we only got to test the Gal System due to time limits. And we figured that the result for Gal System is adequately enough to represent the feasibility of our designed strategy for MTS.</p> |
<p>The whole test process can be divided into three steps. </p> | <p>The whole test process can be divided into three steps. </p> | ||
<h5>Step 1:</h5> | <h5>Step 1:</h5> | ||
| Line 304: | Line 304: | ||
<p> Cultivate two groups of yeasts together. (one is SynⅩ-dUra-416, the other is normal BY4741 MATa) If the MTS has been accomplished (SynⅩ-dUra-416 can become MATα), the two groups of haploids can mate with each other and become diploids. </p> | <p> Cultivate two groups of yeasts together. (one is SynⅩ-dUra-416, the other is normal BY4741 MATa) If the MTS has been accomplished (SynⅩ-dUra-416 can become MATα), the two groups of haploids can mate with each other and become diploids. </p> | ||
<h5>Step 3:</h5> | <h5>Step 3:</h5> | ||
| − | <p> Test the results of mating by PCR method. We designed the primers for both MATa locus and MATα locus. The amplification of both MATa locus and MATα locus indicates that the yeasts | + | <p> Test the results of mating by PCR method. We designed the primers for both MATa locus and MATα locus. The amplification of both MATa locus and MATα locus indicates that the yeasts have turned into diploids, the MTS has been achieved in other words. </p> |
<h4>BACKGROUND</h4> | <h4>BACKGROUND</h4> | ||
| Line 324: | Line 324: | ||
<h5>Artificial Transcription Factors——Z4EV</h5> | <h5>Artificial Transcription Factors——Z4EV</h5> | ||
| − | <p>Thanks to R. Scott McIsaac and Benjamin L. Oakes’s former work, we learned that Z4EV is a kind of fusion protein with three domains | + | <p>Thanks to R. Scott McIsaac and Benjamin L. Oakes’s former work, we learned that Z4EV is a kind of fusion protein with three domains –a DNA binding domain (DBD), an estrogen receptor (ER) and a VP16 activation domain. In the absence of β-estradiol, the ER interacts with Hsp90 chaperone complex and keep the ATF out of the nucleus. This AFT will provide a strong transcriptional activator that is dependent on the presence of β-estradiol. By using a synthetic 4-time-repeated zinc-finger DBD array from the mouse TF Zif268, residual off-target effects have been totally avoided. </p> |
| − | <p>Z4EV (the Z4EV gene has been | + | <p>Z4EV (the Z4EV gene has been introduced into the SynX chromosome of this group of haploids) to strictly control the expression of HO gene. Unlike common β-estradiol-induced or galactose-induced promoters, this modified promoter is designed to be activated only when it is specifically bound with the activated Z4EV factor. </p> |
<div class="zxx_zoom_demo" align="center"> | <div class="zxx_zoom_demo" align="center"> | ||
<script type="text/javascript" src="https://2017.igem.org/Team:Tianjin/Resources/JS:zoom?action=raw&ctype=text/javascript"></script> | <script type="text/javascript" src="https://2017.igem.org/Team:Tianjin/Resources/JS:zoom?action=raw&ctype=text/javascript"></script> | ||
| Line 464: | Line 464: | ||
</p> | </p> | ||
| − | <p> Second, we expect to observe the expression of red fluorescent protein before switch is on. We expect red fluorescent protein to be observed under a fluorescence microscope. And it is desirable that the fluorescence spectrophotometer can measure a high and stable fluorescence value. | + | <p> Second, we expect to observe the expression of red fluorescent protein before the switch is on. We expect red fluorescent protein to be observed under a fluorescence microscope. And it is desirable that the fluorescence spectrophotometer can measure a high and stable fluorescence value. |
</p> | </p> | ||
| Line 516: | Line 516: | ||
<h4>Theoretical Background</h4> | <h4>Theoretical Background</h4> | ||
<h5>Sc2.0 Project</h5> | <h5>Sc2.0 Project</h5> | ||
| − | <p>Synthetic Yeast Genome Project (Sc2.0) is the world’s first synthetic eukaryotic genome project that aims to create a novel, rationalized version of the genome of the yeast species Saccharomyces cerevisiae. On March 10th, 7 articles related to Sc 2.0 were published on Science. As a member of Sc 2.0, YJ lab has completed two synthetic yeast chromosomes, and two articles are published on Science discussing | + | <p>Synthetic Yeast Genome Project (Sc2.0) is the world’s first synthetic eukaryotic genome project that aims to create a novel, rationalized version of the genome of the yeast species Saccharomyces cerevisiae. On March 10th, 7 articles related to Sc 2.0 were published on Science. As a member of Sc 2.0, YJ lab has completed two synthetic yeast chromosomes, and two articles are published on Science discussing challenging but exciting task of building synthetic chromosomes V, X .</p> |
<h5>Cre-<i>loxpsym</i> System</h5> | <h5>Cre-<i>loxpsym</i> System</h5> | ||
| − | <p>As a part of the Sc2.0 Project , yeast chromosomes are targets, named <i>loxPsym</i> sites. <i>loxPsym</i> sites are substrates for Cre-EBD , which is an inducible form of the appropriate site-specific recombinase. Results of the recombination events are dependent on the directionality of the loxp sites. Because of the asymmetry of the loxp sites, when a pair of loxp sites is present in the same DNA strand and they are in opposite orientations, the Cre recombinase will catalyze the inversion of the gene in between the loxp sites; when the loxp sites are in same orientation, this gene will be deleted. Unlike the native directional <i>loxP</i> site, which permits a single orientation for recombination, the synthetic <i>loxPsym</i> site’s symmetry ensures that any pair of sites can recom- bine in either orientation. Then, controlled expression of Cre-EBD | + | <p>As a part of the Sc2.0 Project , yeast chromosomes are targets, named <i>loxPsym</i> sites. <i>loxPsym</i> sites are substrates for Cre-EBD , which is an inducible form of the appropriate site-specific recombinase. Results of the recombination events are dependent on the directionality of the loxp sites. Because of the asymmetry of the loxp sites, when a pair of loxp sites is present in the same DNA strand and they are in opposite orientations, the Cre recombinase will catalyze the inversion of the gene in between the loxp sites; when the loxp sites are in same orientation, this gene will be deleted. Unlike the native directional <i>loxP</i> site, which permits a single orientation for recombination, the synthetic <i>loxPsym</i> site’s symmetry ensures that any pair of sites can recom- bine in either orientation. Then, controlled expression of Cre-EBD leads to deletions and inversions with chromosome segments flanked by <i>loxPsym</i> sites. This characteristic allows more possibilities of recombination on yeast chromosome which lives up to our expectations. </p> |
| Line 589: | Line 589: | ||
| − | <p>What's more, we measure the | + | <p>What's more, we measure the survival rate of optimal strains with original strains <i>synX</i>. After cultivating yeasts in YPD overnight, we take 200μL culture medium into ultrahigh concentration copper ion and cadmium ion solution. Then, plate after 10min, 30min, 1h and 2h. Through counting the number of colonies, we can obtain and compare the curve describing the cell survival rate of optimal strain and <i>synX</i>.</p> |
<div class="zxx_zoom_demo_long_q" align="center"> | <div class="zxx_zoom_demo_long_q" align="center"> | ||
| Line 635: | Line 635: | ||
<p>The characterization of this promoter wasn’t perfectly completed, so we first construct a biosensor based on CUP1 promoter (<a href="http://parts.igem.org/Part:BBa_K2165004">BBa_K2165004</a>) and yEmRFP (<a href="http://parts.igem.org/Part:BBa_K2407012">BBa_K2407012</a>) to improve the characterization and function of this BioBrick.</p> | <p>The characterization of this promoter wasn’t perfectly completed, so we first construct a biosensor based on CUP1 promoter (<a href="http://parts.igem.org/Part:BBa_K2165004">BBa_K2165004</a>) and yEmRFP (<a href="http://parts.igem.org/Part:BBa_K2407012">BBa_K2407012</a>) to improve the characterization and function of this BioBrick.</p> | ||
| − | <p>To improve this promoter, we first want to shorten this promoter with all core sequence retained. Compared with other promoters used in prokaryote, this promoter is much longer, which is not beneficial for BioBricks to easily assembly. Under this condition, we want to shorten its sequence as much as possible without functional changes.</p> | + | <p>To improve this promoter, we first want to shorten this promoter with all core sequence retained. Compared with other promoters used in the prokaryote, this promoter is much longer, which is not beneficial for BioBricks to easily assembly. Under this condition, we want to shorten its sequence as much as possible without functional changes.</p> |
| − | <p>In fact, this promoter is leaky in the absence of inducer. To make it more sensitive and lower the threshold of expression, we chose to transform the promoter with error-prone PCR. After finishing the library, the sensitivity and response | + | <p>In fact, this promoter is leaky in the absence of inducer. To make it more sensitive and lower the threshold of expression, we chose to transform the promoter with error-prone PCR. After finishing the library, the sensitivity and response range of engineered promoters will be characterized by the fluorescence intensity.</p> |
<p>After the detection, we will use Mating Switcher to rapidly open following gene’s expression. We can either overexpress <i>CUP1</i> to enrich copper in the yeast cell or display the Metallothionein on the surface of budding yeast.</p> | <p>After the detection, we will use Mating Switcher to rapidly open following gene’s expression. We can either overexpress <i>CUP1</i> to enrich copper in the yeast cell or display the Metallothionein on the surface of budding yeast.</p> | ||
Revision as of 12:07, 31 October 2017
/* OVERRIDE IGEM SETTINGS */
Design
Background
Human existence on earth is almost impossible without the heavy metals. Even though important to mankind, exposure to them during production, usage and their uncontrolled discharge into the environment has caused lots of hazards to man, other organisms and the environment itself. Heavy metals can enter human tissues and organs via inhalation, diet, and manual handling. As the process of urbanization and industrialization goes deeper and deeper, heavy metal pollution, a noticeable threaten to almost all the creatures, has become an essential problem to solve.
According to our human practice, the situation of heavy metal pollution (copper and cadmium ions) is marked on a world map, and the severity of heavy metal pollution has been increasing all over this map. Places with serious pollution include middle Asia, eastern Asia, southern Europe, and Latin America. In addition, not only fresh water source, but also soil and crops are seriously contaminated by heavy metals. On average, during three out of ten suppers we have, we absorb excess heavy metals over the standard concentration.
Considering the rigorous situation we face, our team decided to design an advanced system for typical toxic heavy metal disposal based on Saccharomyces cerevisiae.