| Line 471: | Line 471: | ||
<p><i>E. coli</i> cells naturally have the C-P lyase pathway which degrades glyphosate into sarcosine. The fact that no formaldehyde was produced when glyphosate was added, but was when sarcosine was added, indicates that we have not overexpressed the C-P lyase pathway enough to produce enough sarcosine for SOX to convert into formaldehyde to be detected. | <p><i>E. coli</i> cells naturally have the C-P lyase pathway which degrades glyphosate into sarcosine. The fact that no formaldehyde was produced when glyphosate was added, but was when sarcosine was added, indicates that we have not overexpressed the C-P lyase pathway enough to produce enough sarcosine for SOX to convert into formaldehyde to be detected. | ||
</br></br> | </br></br> | ||
| − | <p>Due to time constraints, we were unable to produce an in vivo formaldehyde detector variant of the Sensynova framework. Future characterisation of this part would include using the platform customised as a formaldehyde biosensor in order to sense compound produce and therefore creating a biosensor of glyphosate. | + | <p>Due to time constraints, we were unable to produce an <i>in vivo</i> formaldehyde detector variant of the Sensynova framework. Future characterisation of this part would include using the platform customised as a formaldehyde biosensor in order to sense compound produce and therefore creating a biosensor of glyphosate. |
</p> | </p> | ||
| Line 672: | Line 672: | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Conclusions and Future Work </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Conclusions and Future Work </h2> | ||
| − | <p>The results demonstrate that further characterisation needs to be conducted in order to optimise the Arsenic detector variant in the Sensynova platform. However, due to time constraints, we adapted the IPTG framework modelling results to the preliminary experiments conducted for the framework customised as the Arsenic biosensor. In order for future characterisation of this part, the model should be modified in order to guide in vivo efforts accordingly. | + | <p>The results demonstrate that further characterisation needs to be conducted in order to optimise the Arsenic detector variant in the Sensynova platform. However, due to time constraints, we adapted the IPTG framework modelling results to the preliminary experiments conducted for the framework customised as the Arsenic biosensor. In order for future characterisation of this part, the model should be modified in order to guide <i>in vivo</i> efforts accordingly. |
</p> | </p> | ||
| Line 753: | Line 753: | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Conclusions and Future Work </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Conclusions and Future Work </h2> | ||
| − | <p>The results demonstrate that further characterisation needs to be conducted in order to optimise the psicose detector variant in the Sensynova platform however, due to time constraints resulted from synthesis delays, we lacked the time to be able to do so. The preliminary experiments conducted for the framework customised as the psicose biosensor were conducted by following data resulted from the model of the framework customised as the IPTG sensor. In order for future characterisation of this part, the model should be modified in order to guide in vivo efforts accordingly. | + | <p>The results demonstrate that further characterisation needs to be conducted in order to optimise the psicose detector variant in the Sensynova platform however, due to time constraints resulted from synthesis delays, we lacked the time to be able to do so. The preliminary experiments conducted for the framework customised as the psicose biosensor were conducted by following data resulted from the model of the framework customised as the IPTG sensor. In order for future characterisation of this part, the model should be modified in order to guide <i>in vivo</i> efforts accordingly. |
</br></br> | </br></br> | ||
We also lacked the time to co-culture this part with the Sensynova platform's multiple modules in order for the creation of variants for the Evry Paris-Saclay. The part <a href="http://parts.igem.org/Part:BBa_K2205023"> BBa_K2205023</a>, the Evry Paris-Saclay's psicose biosensor system as the detecting unit of the platform, has been submitted to the iGEM registry for future work and characterisation by future teams.</p> | We also lacked the time to co-culture this part with the Sensynova platform's multiple modules in order for the creation of variants for the Evry Paris-Saclay. The part <a href="http://parts.igem.org/Part:BBa_K2205023"> BBa_K2205023</a>, the Evry Paris-Saclay's psicose biosensor system as the detecting unit of the platform, has been submitted to the iGEM registry for future work and characterisation by future teams.</p> | ||
| Line 824: | Line 824: | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Future Work </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Future Work </h2> | ||
| − | <p>Due to time constraints, we lacked the time to synthesise, implement and characterise this part into the Sensynova platform within the lab. Future work on this part would include characterisation in vivo guided by the modelling of the framework when customised as a formaldehyde biosensor and testing against the Sarcosine Oxidase adaptor module currently present in the framework. | + | <p>Due to time constraints, we lacked the time to synthesise, implement and characterise this part into the Sensynova platform within the lab. Future work on this part would include characterisation <i>in vivo</i> guided by the modelling of the framework when customised as a formaldehyde biosensor and testing against the Sarcosine Oxidase adaptor module currently present in the framework. |
</p> | </p> | ||
| Line 1,082: | Line 1,082: | ||
<p>The expression of deGFP was first tested in <i>E. coli</i> cells using an experimental procedure similar to that used in the Interlab study. Cells transformed with pSB1C3-J23100-deGFP were grown in 10 mL LB broth overnight and OD600 nm was measured. Culture was added to 3 separate falcon tubes and made up to 12 mL with LB with chloramphenicol such that the starting OD600 of the culture was approximately 0.02. This set-up was repeated with cells containing an identical plasmid and construct, except sfGFP was in place of deGFP. As a control, untransformed cells were also prepared identically except the LB did not contain chloramphenicol. Tubes with only LB and LB+chloramphenicol were also prepared as blanks. | <p>The expression of deGFP was first tested in <i>E. coli</i> cells using an experimental procedure similar to that used in the Interlab study. Cells transformed with pSB1C3-J23100-deGFP were grown in 10 mL LB broth overnight and OD600 nm was measured. Culture was added to 3 separate falcon tubes and made up to 12 mL with LB with chloramphenicol such that the starting OD600 of the culture was approximately 0.02. This set-up was repeated with cells containing an identical plasmid and construct, except sfGFP was in place of deGFP. As a control, untransformed cells were also prepared identically except the LB did not contain chloramphenicol. Tubes with only LB and LB+chloramphenicol were also prepared as blanks. | ||
</br></br> | </br></br> | ||
| − | The cultures were shake-incubated at 37oC. 300 μL samples from each tube were taken at time points of 15 mins, 2 hours, 4 hours, and 6 hours and stored at 4oC until the end of the experiment. 100 μL of each sample were then added to a 96-well plate. Fluorescence (excitation 485 nm, emission 510 nm) and absorbance (OD600 nm) were measured using a BMG-Labtech fluostar optima plate reader. Fluorescence intensity and grow rates for all three cell types were calculated over time (Figure 3). It was found that while cells expressing sfGFP had a much higher fluorescence intensity than cells expressing deGFP, the growth rate for cells with deGFP was closer to that of untransformed cells. This suggests that in vivo, either deGFP has lower expression than sfGFP, or each molecule of deGFP emits less fluorescence. | + | The cultures were shake-incubated at 37oC. 300 μL samples from each tube were taken at time points of 15 mins, 2 hours, 4 hours, and 6 hours and stored at 4oC until the end of the experiment. 100 μL of each sample were then added to a 96-well plate. Fluorescence (excitation 485 nm, emission 510 nm) and absorbance (OD600 nm) were measured using a BMG-Labtech fluostar optima plate reader. Fluorescence intensity and grow rates for all three cell types were calculated over time (Figure 3). It was found that while cells expressing sfGFP had a much higher fluorescence intensity than cells expressing deGFP, the growth rate for cells with deGFP was closer to that of untransformed cells. This suggests that <i>in vivo</i>, either deGFP has lower expression than sfGFP, or each molecule of deGFP emits less fluorescence. |
</br></br></p> | </br></br></p> | ||
| Line 1,277: | Line 1,277: | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Conclusions and Future Work </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Conclusions and Future Work </h2> | ||
<p> | <p> | ||
| − | The qualitative results detailed above highlight the crucial advantage of our multicellular, modular framework, as it enables each component to be optimised avoiding any extra cloning steps. As each biosensor may be different and require specific designs and optimisation, easily choosing and changing modules and predicting in silico the bacterial community behaviour is essential for the development of new biosensors. | + | The qualitative results detailed above highlight the crucial advantage of our multicellular, modular framework, as it enables each component to be optimised avoiding any extra cloning steps. As each biosensor may be different and require specific designs and optimisation, easily choosing and changing modules and predicting <i>in silico</i> the bacterial community behaviour is essential for the development of new biosensors. |
</br></br> | </br></br> | ||
| Line 1,364: | Line 1,364: | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Implementation </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Implementation </h2> | ||
| − | <p>To prove that our concept of splitting biosensors across multiple cells would work, we designed an IPTG sensor. The design of this system can be found in Figure 4. In this system, LacI is constitutively expressed in the detector cell and represses the production of LasI. When IPTG is added, it binds LacI, preventing repression. Therefore, in the presence of IPTG, LasI will produce C12, our first connector molecule. To determine that our system would work, it was first tested in silico. Details on the model of this system can be found on our <a href="https://2017.igem.org/Team:Newcastle/Model#sim">Modelling page</a>. | + | <p>To prove that our concept of splitting biosensors across multiple cells would work, we designed an IPTG sensor. The design of this system can be found in Figure 4. In this system, LacI is constitutively expressed in the detector cell and represses the production of LasI. When IPTG is added, it binds LacI, preventing repression. Therefore, in the presence of IPTG, LasI will produce C12, our first connector molecule. To determine that our system would work, it was first tested <i>in silico</i>. Details on the model of this system can be found on our <a href="https://2017.igem.org/Team:Newcastle/Model#sim">Modelling page</a>. |
</br></br> | </br></br> | ||
<img src="https://static.igem.org/mediawiki/2017/5/5c/Iptg_framework.jpg" class="img-fluid rounded mx-auto d-block" alt=""> | <img src="https://static.igem.org/mediawiki/2017/5/5c/Iptg_framework.jpg" class="img-fluid rounded mx-auto d-block" alt=""> | ||
| Line 1,416: | Line 1,416: | ||
</center></p> </br> | </center></p> </br> | ||
<p> | <p> | ||
| − | Results from the <a href="https://2017.igem.org/Team:Newcastle/Model#sim">multicellular modelling</a> predicted that the traditionally used 1:1:1 ratio is not the optimal combination for the Sensynova device to work. It is in fact suggested to adopt a higher concentration of the reporter culture compare with the detector and processor. Thus, the framework test was repeated incorporating our in silico simulation data and combining the 3 cell types in ratio 1:1:13 (detectors:processors:reporters). The experiment results, shown in the picture below, confirm the modelling data. There is a consistent discrepancy between IPTG induced and non-induced samples in the 1:1:13 co-cultures, in comparison with the 1:1:1 co-cultures which don't show any difference in presence or absence of IPTG (Figure 9).</p></br> | + | Results from the <a href="https://2017.igem.org/Team:Newcastle/Model#sim">multicellular modelling</a> predicted that the traditionally used 1:1:1 ratio is not the optimal combination for the Sensynova device to work. It is in fact suggested to adopt a higher concentration of the reporter culture compare with the detector and processor. Thus, the framework test was repeated incorporating our <i>in silico</i> simulation data and combining the 3 cell types in ratio 1:1:13 (detectors:processors:reporters). The experiment results, shown in the picture below, confirm the modelling data. There is a consistent discrepancy between IPTG induced and non-induced samples in the 1:1:13 co-cultures, in comparison with the 1:1:1 co-cultures which don't show any difference in presence or absence of IPTG (Figure 9).</p></br> |
<p> | <p> | ||
<img src="https://static.igem.org/mediawiki/2017/0/0d/T--Newcastle--BB_framework_framework_green1_1_13.jpg" class="img-fluid rounded mx-auto d-block" alt=""> | <img src="https://static.igem.org/mediawiki/2017/0/0d/T--Newcastle--BB_framework_framework_green1_1_13.jpg" class="img-fluid rounded mx-auto d-block" alt=""> | ||
| Line 1,467: | Line 1,467: | ||
</table> | </table> | ||
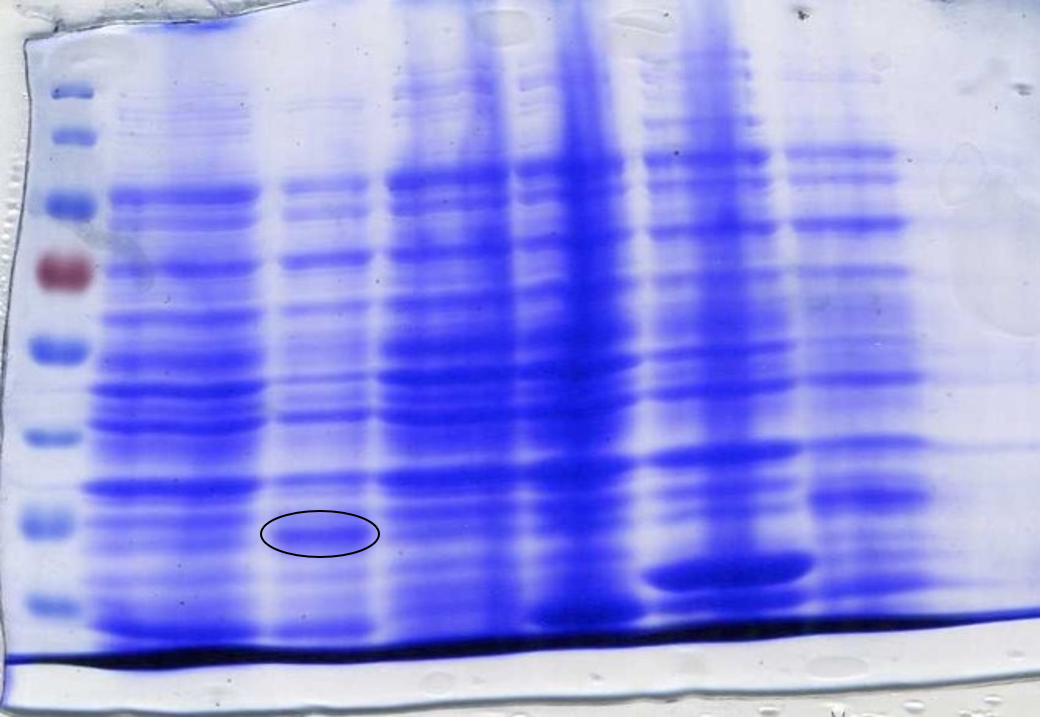
<p>The 3 experiment sets clearly show that the framework is optimised when a higher concentration of cells expressing the reporter device is present (Figures 10, 11, 12, samples labelled 1:1:13). This can be considered as a further validation of our fine-tuning approach using the <a href="https://2017.igem.org/Team:Newcastle/Model#sim">simbiotics model</a> and the previous plate reader experiments. | <p>The 3 experiment sets clearly show that the framework is optimised when a higher concentration of cells expressing the reporter device is present (Figures 10, 11, 12, samples labelled 1:1:13). This can be considered as a further validation of our fine-tuning approach using the <a href="https://2017.igem.org/Team:Newcastle/Model#sim">simbiotics model</a> and the previous plate reader experiments. | ||
| − | Although a background signal is visible in the systems expressing the pink (<a href="http://parts.igem.org/Part:BBa_K2205018">BBa_K2205018</a>)and the sfGPF(<a href="http://parts.igem.org/Part:BBa_K2205015">BBa_K2205015</a>) reporters, the blue reporter (<a href="http://parts.igem.org/Part:BBa_K2205016">BBa_K2205016</a>) due to its lowest background level, constitutes the most suitable reporter module for the Sensynova platform customised as IPTG biosensor. This highlights a crucial advantage of our multicellular, modular framework, which enables each component to be optimised avoiding any extra cloning steps. As each biosensor may be different and require specific designs and optimisation, easily choosing and changing modules and predicting in silico the bacterial community behavior is essential for the development of new biosensors. </p> | + | Although a background signal is visible in the systems expressing the pink (<a href="http://parts.igem.org/Part:BBa_K2205018">BBa_K2205018</a>)and the sfGPF(<a href="http://parts.igem.org/Part:BBa_K2205015">BBa_K2205015</a>) reporters, the blue reporter (<a href="http://parts.igem.org/Part:BBa_K2205016">BBa_K2205016</a>) due to its lowest background level, constitutes the most suitable reporter module for the Sensynova platform customised as IPTG biosensor. This highlights a crucial advantage of our multicellular, modular framework, which enables each component to be optimised avoiding any extra cloning steps. As each biosensor may be different and require specific designs and optimisation, easily choosing and changing modules and predicting <i>in silico</i> the bacterial community behavior is essential for the development of new biosensors. </p> |
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Conclusions and Future Work </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Conclusions and Future Work </h2> | ||
<p>In conclusion, through a comprehensive systematic review a design pattern of four components was identified for synthetic biology biosensors. The components are detection and output devices, with optional processing and adaptor units. Based on this design pattern, a multicellular biosensor development platform was designed in which biosensor components were split between cells and linked by intercellular connectors. | <p>In conclusion, through a comprehensive systematic review a design pattern of four components was identified for synthetic biology biosensors. The components are detection and output devices, with optional processing and adaptor units. Based on this design pattern, a multicellular biosensor development platform was designed in which biosensor components were split between cells and linked by intercellular connectors. | ||
| Line 1,522: | Line 1,522: | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Background Information </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Background Information </h2> | ||
<h4 style="font-family: Rubik; text-align: left; margin-top: 1%"> Cell Free Protein Synthesis Systems </h4> | <h4 style="font-family: Rubik; text-align: left; margin-top: 1%"> Cell Free Protein Synthesis Systems </h4> | ||
| − | <p>Cell free protein synthesis (CFPS) systems are capable of performing transcription and translation of exogenous DNA in vitro. CFPS systems have been in use for many decades (Nirenberg & Matthaei, 1961), however the field of synthetic biology has resulted in a CFPS renaissance (Lu, 2017; Lee & Kim, 2013). Commonly, CFPS systems are based on cell extracts, which provide the transcription/translation machinery, as well as enzymes required to generate ATP required for protein synthesis. | + | <p>Cell free protein synthesis (CFPS) systems are capable of performing transcription and translation of exogenous DNA <i>in vitro</i>. CFPS systems have been in use for many decades (Nirenberg & Matthaei, 1961), however the field of synthetic biology has resulted in a CFPS renaissance (Lu, 2017; Lee & Kim, 2013). Commonly, CFPS systems are based on cell extracts, which provide the transcription/translation machinery, as well as enzymes required to generate ATP required for protein synthesis. |
</br></br> | </br></br> | ||
While CFPS systems have a lot of potential, they also suffer from some drawbacks. Two of the major issues are the large variation in CFPS activity between cell extracts (Katsura, <i>et al</i>., 2017), and the high costs compared to whole cells (although cost have been reduced significantly in the past decade) (Carlson, <i>et al</i>., 2012). These issues can hinder the uptake of CFPS systems as an alternative chassis to whole cells, and as research tools.</p> | While CFPS systems have a lot of potential, they also suffer from some drawbacks. Two of the major issues are the large variation in CFPS activity between cell extracts (Katsura, <i>et al</i>., 2017), and the high costs compared to whole cells (although cost have been reduced significantly in the past decade) (Carlson, <i>et al</i>., 2012). These issues can hinder the uptake of CFPS systems as an alternative chassis to whole cells, and as research tools.</p> | ||
Revision as of 13:30, 1 November 2017
spacefill
spacefill
Our Experimental Results
Below is a diagram of our Sensynova Framework. Clicking on each part of the framework (e.g. detector modules) links to the relevant results.
Alternatively, at the bottom of this page are tabs which will show you results for every part of the project






























































 The
The