| Line 1,117: | Line 1,117: | ||
<h2 style="font-size: 1em"> BioBricks used: <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2205001">BBa_K2205001 (New)</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2205002">BBa_K2205002 (New)</a>, <a href="http://parts.igem.org/Part:BBa_K515105">BBa_K515105 (Imperial College London 2011)</a> </h2> | <h2 style="font-size: 1em"> BioBricks used: <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2205001">BBa_K2205001 (New)</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2205002">BBa_K2205002 (New)</a>, <a href="http://parts.igem.org/Part:BBa_K515105">BBa_K515105 (Imperial College London 2011)</a> </h2> | ||
| + | </br> | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Rationale and Aim </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Rationale and Aim </h2> | ||
| Line 1,123: | Line 1,124: | ||
</br></br> | </br></br> | ||
The aims for this section of the project were: (i) to standardise deGFP as a BioBrick and submit it to the iGEM repository, (ii) to demonstrate expression of deGFP in commercially available cell-free systems, and (iii) to characterise expression of deGFP in <i>E. coli</i> cells.</p> | The aims for this section of the project were: (i) to standardise deGFP as a BioBrick and submit it to the iGEM repository, (ii) to demonstrate expression of deGFP in commercially available cell-free systems, and (iii) to characterise expression of deGFP in <i>E. coli</i> cells.</p> | ||
| + | </br> | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Background Information </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Background Information </h2> | ||
</br> | </br> | ||
<p>deGFP is a modified variant of eGFP developed by Shin and Noireaux which is more efficiently translated in CFPS systems. It was designed by truncating the N-terminal sequence and introducing silent mutations which removed internal ribosome binding like sequences. The C-terminal sequence is also truncated as this has been shown to not be necessary for maximal fluorescence (Li <i>et al</i>. 1997). By removing ribosome binding like sequences, Shin and Noireaux have reduced the amount of incorrect ribosome binding events and hence increased translation efficiency. The length of the protein also contributes to enhanced translation efficiency by reducing the time and resources required for this process to reach completion.</p> | <p>deGFP is a modified variant of eGFP developed by Shin and Noireaux which is more efficiently translated in CFPS systems. It was designed by truncating the N-terminal sequence and introducing silent mutations which removed internal ribosome binding like sequences. The C-terminal sequence is also truncated as this has been shown to not be necessary for maximal fluorescence (Li <i>et al</i>. 1997). By removing ribosome binding like sequences, Shin and Noireaux have reduced the amount of incorrect ribosome binding events and hence increased translation efficiency. The length of the protein also contributes to enhanced translation efficiency by reducing the time and resources required for this process to reach completion.</p> | ||
| + | </br> | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Design Stage </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Design Stage </h2> | ||
| Line 1,208: | Line 1,211: | ||
<p class="legend"><center><strong>Figure 6:</strong> CFPS Expression of J23100-deGFP and J23100-sfGFP Constructs. Time course for increase in fluorescence intensity of CFPS systems expressing GFP constructs over time. Each data point is an average of triplicate results, and error bars show +/- standard error. CFPS system with no DNA (Red) was used as the negative control.</center></p> | <p class="legend"><center><strong>Figure 6:</strong> CFPS Expression of J23100-deGFP and J23100-sfGFP Constructs. Time course for increase in fluorescence intensity of CFPS systems expressing GFP constructs over time. Each data point is an average of triplicate results, and error bars show +/- standard error. CFPS system with no DNA (Red) was used as the negative control.</center></p> | ||
</div> | </div> | ||
| + | </br> | ||
| Line 1,219: | Line 1,223: | ||
</p> | </p> | ||
| + | </br> | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> References </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> References </h2> | ||
| Line 1,240: | Line 1,245: | ||
<h2 style="font-size: 1em"> BioBricks used: <a href="http://parts.igem.org/Part:BBa_K2205016">BBa_K2205016(New)</a>,<a href="http://parts.igem.org/Part:BBa_K2205017">BBa_K2205017(New)</a>,<a href="http://parts.igem.org/Part:BBa_K2205018">BBa_K2205018(New)</a>, <a href="http://parts.igem.org/Part:BBa_K1033915">BBa_K1033915(Uppsala 2013)</a>, <a href="http://parts.igem.org/Part:BBa_K1033925">BBa_K1033925 (Uppsala 2013)</a>, <a href="http://parts.igem.org/Part:BBa_K1033929">BBa_K1033929 (Uppsala 2013)</a> </h2> | <h2 style="font-size: 1em"> BioBricks used: <a href="http://parts.igem.org/Part:BBa_K2205016">BBa_K2205016(New)</a>,<a href="http://parts.igem.org/Part:BBa_K2205017">BBa_K2205017(New)</a>,<a href="http://parts.igem.org/Part:BBa_K2205018">BBa_K2205018(New)</a>, <a href="http://parts.igem.org/Part:BBa_K1033915">BBa_K1033915(Uppsala 2013)</a>, <a href="http://parts.igem.org/Part:BBa_K1033925">BBa_K1033925 (Uppsala 2013)</a>, <a href="http://parts.igem.org/Part:BBa_K1033929">BBa_K1033929 (Uppsala 2013)</a> </h2> | ||
| + | </br> | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Rationale and Aim </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Rationale and Aim </h2> | ||
| Line 1,246: | Line 1,252: | ||
</br></br> | </br></br> | ||
This section of the project is based on testing the modularity of the system by replacing the sfGFP output part of the Sensynova platform design with three different output chromoprotein variants; BBa_K1033929 (aeBlue), BBa_K1033925 (spisPink) and BBa_K1033915 (amajLime).</p> | This section of the project is based on testing the modularity of the system by replacing the sfGFP output part of the Sensynova platform design with three different output chromoprotein variants; BBa_K1033929 (aeBlue), BBa_K1033925 (spisPink) and BBa_K1033915 (amajLime).</p> | ||
| + | </br> | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Background Information </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Background Information </h2> | ||
| Line 1,267: | Line 1,274: | ||
<p>The aeBlue protein is a blue chromoprotein extracted from the basal disk of a beadlet anemone Actinia equine. It was first extracted and characterized by Shkrob <i>et al</i>. 2005 under the name aeCP597 and codon optimised for <i>E. coli</i> by Bioneer Corp. The protein has an absorption maximum at 597nm and a deep blue colour visible to the naked eye. The protein aeBlue has significant sequence homologies with proteins in the GFP family. The coding sequence for this protein was originally submitted to the registry as BBa_K1033916 by the 2012 Uppsala iGEM team.</p> | <p>The aeBlue protein is a blue chromoprotein extracted from the basal disk of a beadlet anemone Actinia equine. It was first extracted and characterized by Shkrob <i>et al</i>. 2005 under the name aeCP597 and codon optimised for <i>E. coli</i> by Bioneer Corp. The protein has an absorption maximum at 597nm and a deep blue colour visible to the naked eye. The protein aeBlue has significant sequence homologies with proteins in the GFP family. The coding sequence for this protein was originally submitted to the registry as BBa_K1033916 by the 2012 Uppsala iGEM team.</p> | ||
<img src="https://static.igem.org/mediawiki/2017/1/1c/T--Newcastle--Lais--Blue.png" class="img-fluid border border-dark rounded" style="margin: 2%; max-width: 70%"> | <img src="https://static.igem.org/mediawiki/2017/1/1c/T--Newcastle--Lais--Blue.png" class="img-fluid border border-dark rounded" style="margin: 2%; max-width: 70%"> | ||
| + | </br> | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Design Stage </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Design Stage </h2> | ||
| Line 1,301: | Line 1,309: | ||
</br></br> | </br></br> | ||
Stared colonies picked from streaked plates and cultures were prepared for <a href="https://static.igem.org/mediawiki/2017/e/e1/T--Newcastle--Miniprep.pdf">miniprepping</a>. DNA samples were then sent off for <a href="https://www.gatc-biotech.com/en/index.html">sequencing</a> to ensure that the constructs were correct. Sequencing data can be found on the <a href="https://2017.igem.org/Team:Newcastle/Parts">Parts page</a>.</p> | Stared colonies picked from streaked plates and cultures were prepared for <a href="https://static.igem.org/mediawiki/2017/e/e1/T--Newcastle--Miniprep.pdf">miniprepping</a>. DNA samples were then sent off for <a href="https://www.gatc-biotech.com/en/index.html">sequencing</a> to ensure that the constructs were correct. Sequencing data can be found on the <a href="https://2017.igem.org/Team:Newcastle/Parts">Parts page</a>.</p> | ||
| + | </br> | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Characterisation </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Characterisation </h2> | ||
| Line 1,361: | Line 1,370: | ||
Although a background signal is visible in the systems expressing the pink (<a href="http://parts.igem.org/Part:BBa_K2205018">BBa_K2205018</a>) and the sfGPF (<a href="http://parts.igem.org/Part:BBa_K2205015">BBa_K2205015</a>) reporters, the blue reporter (<a href="http://parts.igem.org/Part:BBa_K2205016">BBa_K2205016</a>) due to its lowest background level, constitutes the most suitable reporter module for the Sensynova platform when customised as IPTG biosensor. | Although a background signal is visible in the systems expressing the pink (<a href="http://parts.igem.org/Part:BBa_K2205018">BBa_K2205018</a>) and the sfGPF (<a href="http://parts.igem.org/Part:BBa_K2205015">BBa_K2205015</a>) reporters, the blue reporter (<a href="http://parts.igem.org/Part:BBa_K2205016">BBa_K2205016</a>) due to its lowest background level, constitutes the most suitable reporter module for the Sensynova platform when customised as IPTG biosensor. | ||
</p> | </p> | ||
| + | </br> | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Conclusions and Future Work </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Conclusions and Future Work </h2> | ||
| Line 1,390: | Line 1,400: | ||
<h2 style="font-size: 1em"> BioBricks used: <a href="http://parts.igem.org/Part:BBa_K2205009">BBa_K2205009(New)</a>, <a href="http://parts.igem.org/Part:BBa_K2205012">BBa_K2205012(New)</a>, <a href="http://parts.igem.org/Part:BBa_K2205015">BBa_K2205015(New)</a>, <a href="http://parts.igem.org/Part:BBa_K2205016">BBa_K2205016(New)</a> and <a href="http://parts.igem.org/Part:BBa_K2205018">BBa_K2205018(New)</a>. | <h2 style="font-size: 1em"> BioBricks used: <a href="http://parts.igem.org/Part:BBa_K2205009">BBa_K2205009(New)</a>, <a href="http://parts.igem.org/Part:BBa_K2205012">BBa_K2205012(New)</a>, <a href="http://parts.igem.org/Part:BBa_K2205015">BBa_K2205015(New)</a>, <a href="http://parts.igem.org/Part:BBa_K2205016">BBa_K2205016(New)</a> and <a href="http://parts.igem.org/Part:BBa_K2205018">BBa_K2205018(New)</a>. | ||
| + | </br> | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Rationale and Aim </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Rationale and Aim </h2> | ||
| Line 1,395: | Line 1,406: | ||
<p> Biosensors, synthetic systems designed to detect and respond to a target analyte, are a common application of synthetic biology. However, the production and screening of multiple biosensor system variants is hindered by the inefficiency and specificity of the gene assembly techniques used. The production of circuit variants is important in biosensor production, as sensitivity to target molecules must be tuned. </br></p> | <p> Biosensors, synthetic systems designed to detect and respond to a target analyte, are a common application of synthetic biology. However, the production and screening of multiple biosensor system variants is hindered by the inefficiency and specificity of the gene assembly techniques used. The production of circuit variants is important in biosensor production, as sensitivity to target molecules must be tuned. </br></p> | ||
<p><b>Aim:</b> To develop a multicellular biosensor development platform which utilises cell-mixing, as opposed to genetic re-engineering, to construct biosensor variants.</p> | <p><b>Aim:</b> To develop a multicellular biosensor development platform which utilises cell-mixing, as opposed to genetic re-engineering, to construct biosensor variants.</p> | ||
| + | </br> | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Background Information </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Background Information </h2> | ||
| Line 1,428: | Line 1,440: | ||
</br></p> | </br></p> | ||
<p> The splitting of biosensor components into separate cells may have additional advantages besides ease of variant production. Goni-Moreno <i>et al</i>. (2011) have previously suggested that the use of synthetic quorum sensing circuits enables each cell to be considered an independent logic gate, which may rectify the “fuzzy logic” seen in some biosensors, where stochastic cellular processes may produce false positive results. Quorum sensing has also been previously used to synchronise gene expressions, leading to reduced variability within a population (Danino <i>et al</i>., 2010).</p> | <p> The splitting of biosensor components into separate cells may have additional advantages besides ease of variant production. Goni-Moreno <i>et al</i>. (2011) have previously suggested that the use of synthetic quorum sensing circuits enables each cell to be considered an independent logic gate, which may rectify the “fuzzy logic” seen in some biosensors, where stochastic cellular processes may produce false positive results. Quorum sensing has also been previously used to synchronise gene expressions, leading to reduced variability within a population (Danino <i>et al</i>., 2010).</p> | ||
| − | + | </br> | |
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Preliminary Experiment </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Preliminary Experiment </h2> | ||
</br> | </br> | ||
<p>In order to support our theory that genetic assembly is the rate limiting step in biosensor development, we attempted to assemble a simple GFP producing system using three engineering techniques: BioBrick, Gibson and Golden Gate. Further information about this experiment can be found on our <a href="https://2017.igem.org/Team:Newcastle/InterLab">interlab page</a> . Gibson was the only successful technique we trailed, however, Gibson assembly is not an ideal method for circuit variant production due the the specificity of the overlapping regions: For example, to assemble ten genetic parts into all possible orders would require the use of 90 different overlapping sequences (Ellis <i>et al</i>., 2011). Therefore, the ability to generate circuit variants without the need for further genetic engineering would be useful.</p> | <p>In order to support our theory that genetic assembly is the rate limiting step in biosensor development, we attempted to assemble a simple GFP producing system using three engineering techniques: BioBrick, Gibson and Golden Gate. Further information about this experiment can be found on our <a href="https://2017.igem.org/Team:Newcastle/InterLab">interlab page</a> . Gibson was the only successful technique we trailed, however, Gibson assembly is not an ideal method for circuit variant production due the the specificity of the overlapping regions: For example, to assemble ten genetic parts into all possible orders would require the use of 90 different overlapping sequences (Ellis <i>et al</i>., 2011). Therefore, the ability to generate circuit variants without the need for further genetic engineering would be useful.</p> | ||
| + | </br> | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Design Stage </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Design Stage </h2> | ||
| Line 1,455: | Line 1,468: | ||
<p> We propose that splitting these modular biosensor components into different cells, as shown below, and co-culturing the cells together, will greatly reduce the complexity of biosensor circuit development. </p> | <p> We propose that splitting these modular biosensor components into different cells, as shown below, and co-culturing the cells together, will greatly reduce the complexity of biosensor circuit development. </p> | ||
| + | </br> | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Implementation </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Implementation </h2> | ||
| Line 1,470: | Line 1,484: | ||
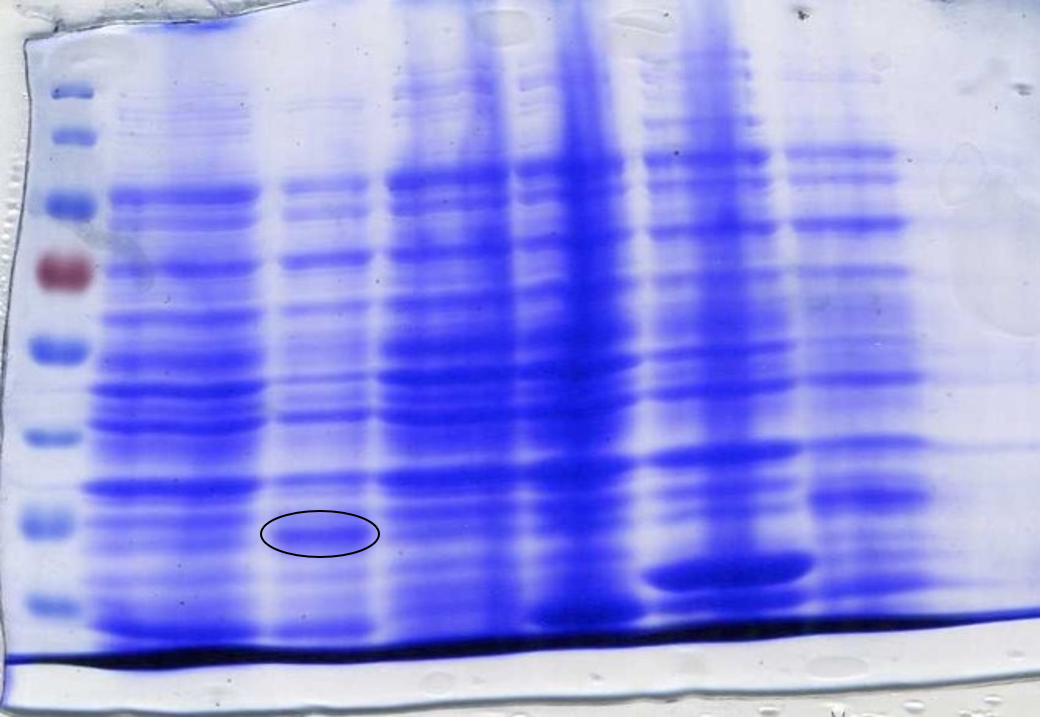
<p> | <p> | ||
<center><b>Figure 5:</b> Colony PCR confirming the correct integration of the biosensor fragments into the vector. </center></p> | <center><b>Figure 5:</b> Colony PCR confirming the correct integration of the biosensor fragments into the vector. </center></p> | ||
| − | + | </br> | |
| Line 1,564: | Line 1,578: | ||
<p>The 3 experiment sets clearly show that the framework is optimised when a higher concentration of cells expressing the reporter device is present (Figures 10, 11, 12, samples labelled 1:1:13). This can be considered as a further validation of our fine-tuning approach using the <a href="https://2017.igem.org/Team:Newcastle/Model#sim">simbiotics model</a> and the previous plate reader experiments. | <p>The 3 experiment sets clearly show that the framework is optimised when a higher concentration of cells expressing the reporter device is present (Figures 10, 11, 12, samples labelled 1:1:13). This can be considered as a further validation of our fine-tuning approach using the <a href="https://2017.igem.org/Team:Newcastle/Model#sim">simbiotics model</a> and the previous plate reader experiments. | ||
Although a background signal is visible in the systems expressing the pink (<a href="http://parts.igem.org/Part:BBa_K2205018">BBa_K2205018</a>)and the sfGPF(<a href="http://parts.igem.org/Part:BBa_K2205015">BBa_K2205015</a>) reporters, the blue reporter (<a href="http://parts.igem.org/Part:BBa_K2205016">BBa_K2205016</a>) due to its lowest background level, constitutes the most suitable reporter module for the Sensynova platform customised as IPTG biosensor. This highlights a crucial advantage of our multicellular, modular framework, which enables each component to be optimised avoiding any extra cloning steps. As each biosensor may be different and require specific designs and optimisation, easily choosing and changing modules and predicting <i>in silico</i> the bacterial community behavior is essential for the development of new biosensors. </p> | Although a background signal is visible in the systems expressing the pink (<a href="http://parts.igem.org/Part:BBa_K2205018">BBa_K2205018</a>)and the sfGPF(<a href="http://parts.igem.org/Part:BBa_K2205015">BBa_K2205015</a>) reporters, the blue reporter (<a href="http://parts.igem.org/Part:BBa_K2205016">BBa_K2205016</a>) due to its lowest background level, constitutes the most suitable reporter module for the Sensynova platform customised as IPTG biosensor. This highlights a crucial advantage of our multicellular, modular framework, which enables each component to be optimised avoiding any extra cloning steps. As each biosensor may be different and require specific designs and optimisation, easily choosing and changing modules and predicting <i>in silico</i> the bacterial community behavior is essential for the development of new biosensors. </p> | ||
| + | </br> | ||
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Conclusions and Future Work </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> Conclusions and Future Work </h2> | ||
<p>In conclusion, through a comprehensive systematic review a design pattern of four components was identified for synthetic biology biosensors. The components are detection and output devices, with optional processing and adaptor units. Based on this design pattern, a multicellular biosensor development platform was designed in which biosensor components were split between cells and linked by intercellular connectors. | <p>In conclusion, through a comprehensive systematic review a design pattern of four components was identified for synthetic biology biosensors. The components are detection and output devices, with optional processing and adaptor units. Based on this design pattern, a multicellular biosensor development platform was designed in which biosensor components were split between cells and linked by intercellular connectors. | ||
| Line 1,572: | Line 1,587: | ||
</br> | </br> | ||
<p><b>The next step. </b>Another advantage to the bypassing of gene assembly enabled by our platform is the increased ability to automate system construction. Microfludic systems are those which control the movement of small volumes of liquids (10–9 to 10–18 litres) using a variety of methods, which may be used to perform biological experiments. These devices have a number of advantages over traditional, manual, lab methods. They only use a small amount of liquid, which means less reagents are consumed and the time taken to perform experiments is reduced. These small amounts of liquids are easier to manipulate than larger volumes, meaning there is greater control over reactions resulting in a high degree of sensitivity (Whitesides, 2006). However, many devices do not have the ability to control temperature, which is important for many methods of gene assembly. Cell mixing, as opposed to gene fragment assembly, is more suited to automation on these platforms, as there is no requirement for precise temperature control. Also, the increased control over small volumes of reagents allows the screening of precise cell ratios. Additionally, programs are in development for the automation of protocols on microfluidic, which will allow the rapid combination of a number of variant biosensor components. To utilise this advantage, we developed <a href="https://2017.igem.org/Team:Newcastle/Model#mf ">software</a> for the simulation of microfludics experiments </p> | <p><b>The next step. </b>Another advantage to the bypassing of gene assembly enabled by our platform is the increased ability to automate system construction. Microfludic systems are those which control the movement of small volumes of liquids (10–9 to 10–18 litres) using a variety of methods, which may be used to perform biological experiments. These devices have a number of advantages over traditional, manual, lab methods. They only use a small amount of liquid, which means less reagents are consumed and the time taken to perform experiments is reduced. These small amounts of liquids are easier to manipulate than larger volumes, meaning there is greater control over reactions resulting in a high degree of sensitivity (Whitesides, 2006). However, many devices do not have the ability to control temperature, which is important for many methods of gene assembly. Cell mixing, as opposed to gene fragment assembly, is more suited to automation on these platforms, as there is no requirement for precise temperature control. Also, the increased control over small volumes of reagents allows the screening of precise cell ratios. Additionally, programs are in development for the automation of protocols on microfluidic, which will allow the rapid combination of a number of variant biosensor components. To utilise this advantage, we developed <a href="https://2017.igem.org/Team:Newcastle/Model#mf ">software</a> for the simulation of microfludics experiments </p> | ||
| − | + | </br> | |
<h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> References </h2> | <h2 style="font-family: Rubik; text-align: left; margin-top: 1%"> References </h2> | ||
</br> | </br> | ||
Revision as of 17:41, 1 November 2017
spacefill
spacefill
Our Experimental Results
Below is a diagram of our Sensynova Framework. Clicking on each part of the framework (e.g. detector modules) links to the relevant results.
Alternatively, at the bottom of this page are tabs which will show you results for every part of the project






























































 The
The