| Line 546: | Line 546: | ||
<h4>Modeling of the expression of Gene(Cd)</h4> | <h4>Modeling of the expression of Gene(Cd)</h4> | ||
| − | + | <hr> | |
<p>The process is similar to the process of the expression of gene(Cu).</p> | <p>The process is similar to the process of the expression of gene(Cu).</p> | ||
| Line 564: | Line 564: | ||
<h4>Simulation results</h4> | <h4>Simulation results</h4> | ||
| − | + | <hr> | |
<p>The simulation results of the model are shown in the figure above, and the concentration of Cu protein in the initial stage of simulation is still increased due to the presence of mRNA. After the reduction of DNA (Cu) and degradation of mRNA (Cu), Cu protein will gradually disappear. Cr protein is very slow in the beginning due to lack of initial DNA (Cr), and then the Cr protein gradually increases due to the accumulation of DNA (Cr), and then the number gradually becomes stable.</p> | <p>The simulation results of the model are shown in the figure above, and the concentration of Cu protein in the initial stage of simulation is still increased due to the presence of mRNA. After the reduction of DNA (Cu) and degradation of mRNA (Cu), Cu protein will gradually disappear. Cr protein is very slow in the beginning due to lack of initial DNA (Cr), and then the Cr protein gradually increases due to the accumulation of DNA (Cr), and then the number gradually becomes stable.</p> | ||
<div class="zxx_zoom_demo" align="center"> | <div class="zxx_zoom_demo" align="center"> | ||
| Line 604: | Line 604: | ||
</div> | </div> | ||
<div class="collapse-card__body"> | <div class="collapse-card__body"> | ||
| − | <h4>Overview</h4> | + | <h4>Overview</h4><hr> |
<p> In our experiment we use engineered yeast cells to absorb and enrich heavy metals such as cooper and cadmium. At first, heavy metal ions diffuse into the cell surface from the liquid phase body, and then heavy metal ions are combined with those heavy metal-treated proteins inside the yeast cells.</p> | <p> In our experiment we use engineered yeast cells to absorb and enrich heavy metals such as cooper and cadmium. At first, heavy metal ions diffuse into the cell surface from the liquid phase body, and then heavy metal ions are combined with those heavy metal-treated proteins inside the yeast cells.</p> | ||
| Line 620: | Line 620: | ||
| − | <h4>Summary</h4> | + | <h4>Summary</h4><hr> |
<p>Treating heavy metal pollution by means of biosorption is a complicated process. First, it is very meaningful to study the growth of yeast in heavy metal ions solution. Considering that the toxic effects of heavy metal ions on yeast can’t be ignored, we use the matrix inhibition growth model to simulate the growth kinetics of yeast in heavy metal ions solution. Next, we decide to study the process of biological adsorption from the thermodynamic and kinetic point of view. In terms of thermodynamics, we use the basic thermodynamic function to explain the adsorption process, and the conclusions can guide further optimization of the biosorption in addition, different static adsorption models are used to simulate the adsorption process, and the conclusions are able to explain the mechanism of the part of the biosorption process. Then we discuss the change of heavy metal ions with time in the process of biosorption from the point of view of dynamics, and compare with the actual measured data.</p> | <p>Treating heavy metal pollution by means of biosorption is a complicated process. First, it is very meaningful to study the growth of yeast in heavy metal ions solution. Considering that the toxic effects of heavy metal ions on yeast can’t be ignored, we use the matrix inhibition growth model to simulate the growth kinetics of yeast in heavy metal ions solution. Next, we decide to study the process of biological adsorption from the thermodynamic and kinetic point of view. In terms of thermodynamics, we use the basic thermodynamic function to explain the adsorption process, and the conclusions can guide further optimization of the biosorption in addition, different static adsorption models are used to simulate the adsorption process, and the conclusions are able to explain the mechanism of the part of the biosorption process. Then we discuss the change of heavy metal ions with time in the process of biosorption from the point of view of dynamics, and compare with the actual measured data.</p> | ||
| − | <h4>Yeast growth model</h4> | + | <h4>Yeast growth model</h4><hr> |
<p>Heavy metal ions inhibit the growth of yeast. In order to describe the kinetics of cell growth accurately,these factors should be taken into account. Unlike the traditional Monod equation, Andrew equation takes the presence of matrix anticompetitive inhibition into consideration.</p> | <p>Heavy metal ions inhibit the growth of yeast. In order to describe the kinetics of cell growth accurately,these factors should be taken into account. Unlike the traditional Monod equation, Andrew equation takes the presence of matrix anticompetitive inhibition into consideration.</p> | ||
<p> | <p> | ||
| Line 659: | Line 659: | ||
<p> </p> | <p> </p> | ||
<p>Taking the presence of matrix inhibition into consideration, when the concentration of heavy metal ions is low, the cell growth rate increases with the increase of heavy metal ions concentration and could reach the maximum value. When the heavy metal concentration continues to increase, the cell growth rate decreases. But when there is no matrix inhibition (Monod equation), the cell growth rate increases with the concentration of the matrix until it approaches the maximum value \({\mu _{\max }}\)</p> | <p>Taking the presence of matrix inhibition into consideration, when the concentration of heavy metal ions is low, the cell growth rate increases with the increase of heavy metal ions concentration and could reach the maximum value. When the heavy metal concentration continues to increase, the cell growth rate decreases. But when there is no matrix inhibition (Monod equation), the cell growth rate increases with the concentration of the matrix until it approaches the maximum value \({\mu _{\max }}\)</p> | ||
| − | <h4>Thermodynamics of Adsorption of Heavy Metals</h4> | + | <h4>Thermodynamics of Adsorption of Heavy Metals</h4><hr> |
<p>In order to study the ability of yeast to treat the pollution of heavy metal, we quantify the process of biosorption from a thermodynamic point of view. Therefore the conception of separation constant Kc is introduced to measure the equilibrium concentration ratio of intracellular and liquid heavy metal ions. </p> | <p>In order to study the ability of yeast to treat the pollution of heavy metal, we quantify the process of biosorption from a thermodynamic point of view. Therefore the conception of separation constant Kc is introduced to measure the equilibrium concentration ratio of intracellular and liquid heavy metal ions. </p> | ||
<p>\[{K_c} = \frac{{{C_i}}}{{{C_l}}}\]</p> | <p>\[{K_c} = \frac{{{C_i}}}{{{C_l}}}\]</p> | ||
| Line 691: | Line 691: | ||
<p>If \(\Delta H\) > 0,the absorption process can be judged as an endothermic process,vice versa. Besides, the size of the enthalpy variable value can also be used to distinguish between physical adsorption and chemical adsorption. (\Delta S\)>0 indicates that the molecular disorder increases during this adsorption process, and vice versa. (\Delta G\) <0 means that the adsorption process can be carried out spontaneously.</p> | <p>If \(\Delta H\) > 0,the absorption process can be judged as an endothermic process,vice versa. Besides, the size of the enthalpy variable value can also be used to distinguish between physical adsorption and chemical adsorption. (\Delta S\)>0 indicates that the molecular disorder increases during this adsorption process, and vice versa. (\Delta G\) <0 means that the adsorption process can be carried out spontaneously.</p> | ||
<h4>Model of Adsorption process</h4> | <h4>Model of Adsorption process</h4> | ||
| + | <hr> | ||
<p>Static adsorption<br> | <p>Static adsorption<br> | ||
Define the equilibrium adsorption capacity of yeast for heavy metal ions q</p> | Define the equilibrium adsorption capacity of yeast for heavy metal ions q</p> | ||
| Line 752: | Line 753: | ||
<p>Scatchard curve is</p> | <p>Scatchard curve is</p> | ||
<p>\[\frac{{[MX]}}{{{M_e}}} = K({X_0} - [MX])\]</p> | <p>\[\frac{{[MX]}}{{{M_e}}} = K({X_0} - [MX])\]</p> | ||
| − | <h4> Biomass adsorption kinetics</h4> | + | <h4> Biomass adsorption kinetics</h4><hr> |
<p>The biosorption process can be divided into two stages. The first stage occurs on the cell wall surface, and is mainly about the physical adsorption and ion exchange process which is going very fast. The second stage, also known as active adsorption,is mainly about chemical adsorption, and metal ions at this stage can be transported through the active into the cell. This stage consumes the energy generated by cell metabolism, which is carried out very slowly.<br> | <p>The biosorption process can be divided into two stages. The first stage occurs on the cell wall surface, and is mainly about the physical adsorption and ion exchange process which is going very fast. The second stage, also known as active adsorption,is mainly about chemical adsorption, and metal ions at this stage can be transported through the active into the cell. This stage consumes the energy generated by cell metabolism, which is carried out very slowly.<br> | ||
Puranik and Paknikar describe the adsorption kinetics quantitatively in mathematical models. This mathematical model is based on the following two assumptions: | Puranik and Paknikar describe the adsorption kinetics quantitatively in mathematical models. This mathematical model is based on the following two assumptions: | ||
| Line 815: | Line 816: | ||
With this goal, the first thing we needed to do is using computational method simulate the biological process and figure out whether our design is feasible. | With this goal, the first thing we needed to do is using computational method simulate the biological process and figure out whether our design is feasible. | ||
</p> | </p> | ||
| − | <h4>The kinetic model in the single cell </h4> | + | <h4>The kinetic model in the single cell </h4><hr> |
<p>The first of these biological processes is the expression of transcription factor ACE1 which contains two steps: transcription and translation.<br> | <p>The first of these biological processes is the expression of transcription factor ACE1 which contains two steps: transcription and translation.<br> | ||
The transcription step can be described as following ODEs (ordinary differential equations):<br> | The transcription step can be described as following ODEs (ordinary differential equations):<br> | ||
| Line 862: | Line 863: | ||
\(D\)is thickness of absorption vessel;<br> | \(D\)is thickness of absorption vessel;<br> | ||
\(E\)is moral factor of fluorescence microplate.<br> | \(E\)is moral factor of fluorescence microplate.<br> | ||
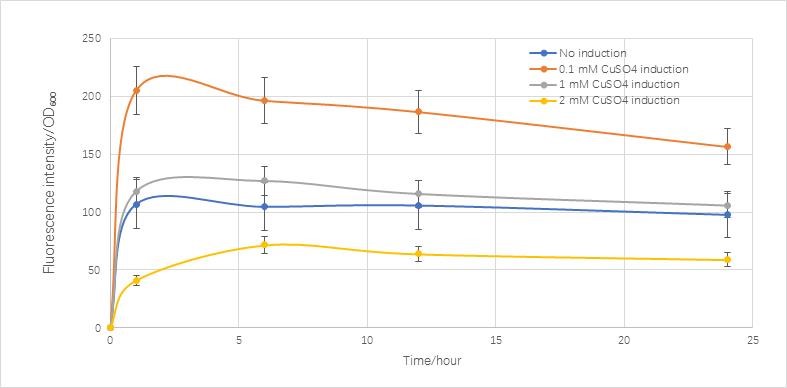
| − | <h4>The Result and analysis of modeling</h4> | + | <h4>The Result and analysis of modeling</h4><hr> |
<p>These constants above can be found in the reference. | <p>These constants above can be found in the reference. | ||
| Line 914: | Line 915: | ||
<p> | <p> | ||
Compared with the real condition we measured,it is very similar to the real condition except in the final stage the real condition concentration is going to decrease.</p> | Compared with the real condition we measured,it is very similar to the real condition except in the final stage the real condition concentration is going to decrease.</p> | ||
| − | <h4>SUMMARY</h4> | + | <h4>SUMMARY</h4> <hr> |
<p>We construct the model of RFP expression in the single cell stage with molecular analysis. The result is similar with the real condition. This model can help us to confirm that the CUP1 promoter is effective to be induced by copper ions. It also can help us to confine the best concentration of copper ions to induce and the maximum expression quantity in a certain copper ions concentration. | <p>We construct the model of RFP expression in the single cell stage with molecular analysis. The result is similar with the real condition. This model can help us to confirm that the CUP1 promoter is effective to be induced by copper ions. It also can help us to confine the best concentration of copper ions to induce and the maximum expression quantity in a certain copper ions concentration. | ||
</p> | </p> | ||
| Line 936: | Line 937: | ||
<div class="collapse-card__body"> | <div class="collapse-card__body"> | ||
<p>This part is not common in modeling, because this part is not a model of our biological system to simulate and predict its behavior. What we do in this part is an assessment of its value and scope of use of our biological systems. In addition, we get a preliminary database of copper and cadmium pollution in the world through the data collection, which is also within the scope of modeling. In this part, we work with teammates who are responsible for human practice to carry out the main work of data collection and map processing. Because different countries and regions have different monitoring and treatment methods for copper and cadmium pollution, and many countries and regions even lack of monitoring and treatment methods of copper and cadmium pollution, we spent a lot of time to collect data. Even so, we did not collect enough data to conduct detailed data analysis of copper and cadmium pollution worldwide. Nevertheless, our existing work can still lead to some preliminary and regional results for the pollution of copper and cadmium.</p> | <p>This part is not common in modeling, because this part is not a model of our biological system to simulate and predict its behavior. What we do in this part is an assessment of its value and scope of use of our biological systems. In addition, we get a preliminary database of copper and cadmium pollution in the world through the data collection, which is also within the scope of modeling. In this part, we work with teammates who are responsible for human practice to carry out the main work of data collection and map processing. Because different countries and regions have different monitoring and treatment methods for copper and cadmium pollution, and many countries and regions even lack of monitoring and treatment methods of copper and cadmium pollution, we spent a lot of time to collect data. Even so, we did not collect enough data to conduct detailed data analysis of copper and cadmium pollution worldwide. Nevertheless, our existing work can still lead to some preliminary and regional results for the pollution of copper and cadmium.</p> | ||
| − | <h4>Data collection</h4> | + | <h4>Data collection</h4><hr> |
<p>As the United States for the detection and processing of pollution is better, we firstly start from the United States to collect data. We find the National Aquatic Resource Surveys in the EPA (United States Environmental Protection Agency) website. There are four national assessments in NARS: National Coastal Condition Assessment (NCCA), National Lakes Assessment (NLA), National Rivers and Streams Assessment (NRSA), National Wetland Condition Assessment (NWCA). Only the NWCA has the data of copper and cadmium, so we use it as one of the data sources of our maps. The methods and standards used in NWCA are shown in EPA websites.</p> | <p>As the United States for the detection and processing of pollution is better, we firstly start from the United States to collect data. We find the National Aquatic Resource Surveys in the EPA (United States Environmental Protection Agency) website. There are four national assessments in NARS: National Coastal Condition Assessment (NCCA), National Lakes Assessment (NLA), National Rivers and Streams Assessment (NRSA), National Wetland Condition Assessment (NWCA). Only the NWCA has the data of copper and cadmium, so we use it as one of the data sources of our maps. The methods and standards used in NWCA are shown in EPA websites.</p> | ||
<p>The methods we used to collect European data is similar to that of the United States. We use the FOREGS-EuroGeoSurveys Geochemical Baseline Database as one of data sources of our maps. The methods and standards used are shown in FOREGS-EuroGeoSurveys Geochemical Baseline Database websites.<p> | <p>The methods we used to collect European data is similar to that of the United States. We use the FOREGS-EuroGeoSurveys Geochemical Baseline Database as one of data sources of our maps. The methods and standards used are shown in FOREGS-EuroGeoSurveys Geochemical Baseline Database websites.<p> | ||
| Line 953: | Line 954: | ||
| − | <h4>Map Drawing</h4> | + | <h4>Map Drawing</h4><hr> |
<p>We stored data we collected as .mat file in MATLAB, and then we use the worldmap and our map drawing command to draw our Global Cu and Cd Pollution Map.</p> | <p>We stored data we collected as .mat file in MATLAB, and then we use the worldmap and our map drawing command to draw our Global Cu and Cd Pollution Map.</p> | ||
| Line 1,027: | Line 1,028: | ||
<p style="text-align:center">The pollution of copper worldwide</p> | <p style="text-align:center">The pollution of copper worldwide</p> | ||
| − | <h4>Analysis of Global Cu and Cd Pollution Map</h4> | + | <h4>Analysis of Global Cu and Cd Pollution Map</h4><hr> |
<p>From the pollution map we can see the following:<br> | <p>From the pollution map we can see the following:<br> | ||
Revision as of 12:24, 1 November 2017
/* OVERRIDE IGEM SETTINGS */
Model
Overview
The models we built included five parts. First, We established a bistable model to analyze the feasibility of functional switch of cells. Then, We used software to model and simulate the functional switch process after cell mating. And we built a RFP expression model to simulate the expression RFP in yeast cells with CUP1 promoter. What’s more, we constructed a model of adsorption to simulate the combination of heavy metal ions and heavy metal-treated proteins inside the yeast cells. Finally, we worked with teammates of human practice to draw the Global Cu and Cd Pollution Map and established a preliminary World Copper and Cadmium Pollution Database.