| Line 1: | Line 1: | ||
{{Aix-Marseille|title=KILL XYL|toc=__TOC__}} | {{Aix-Marseille|title=KILL XYL|toc=__TOC__}} | ||
| − | + | [[File:T--Aix-Marseille--KX.png|600px|center|KILL XYL project.]] | |
| + | |||
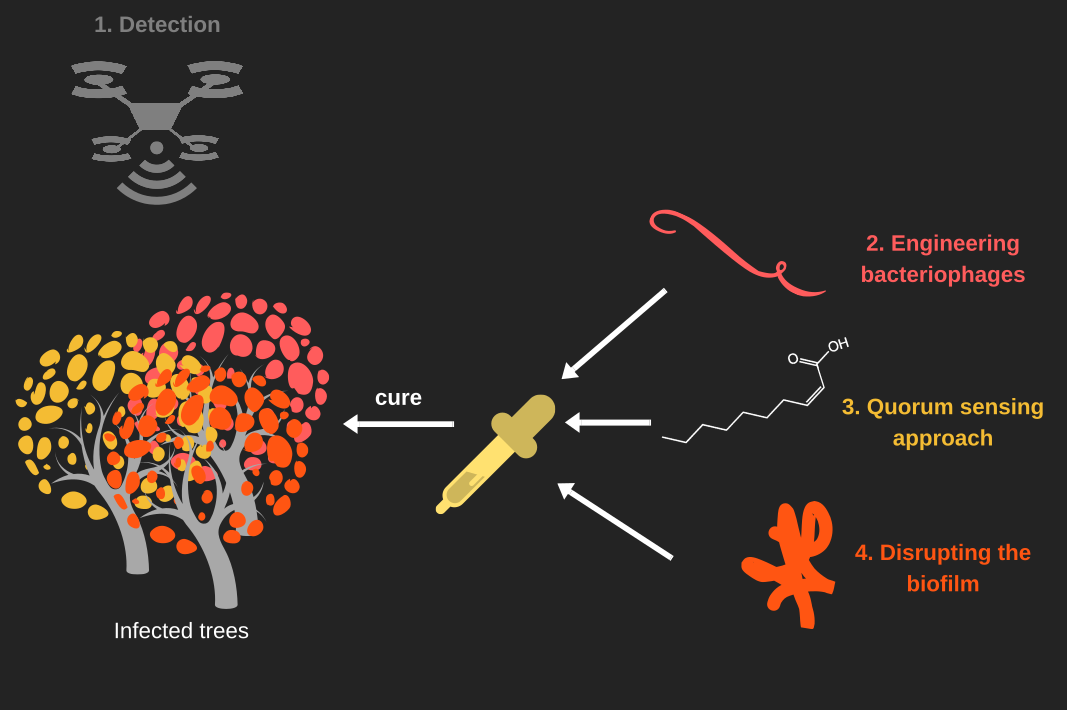
| + | Our project, '''KILL XYL''' will be a cure against the disease caused by the plant pathogen [[Team:Aix-Marseille/Xylella_fastidiosa|''Xylella fastidiosa'']]. This disease currently causes the loss of thousands of hectares of European crops and no extensive cure exists against it. | ||
| − | [[Team:Aix-Marseille/ | + | At Aix-Marseille University we thought about a solution that enclose many aspect of the cure. First, we wanted to improve the [[Team:Aix-Marseille/Hardware|detection]] of the disease. To do so we use a NDVI camera that will help us to see if the plant do photosynthesis or not. If the plant is heavily affected by [[Team:Aix-Marseille/Xylella_fastidiosa|''Xylella fastidiosa'']], it support a hydric stress that stop the photosynthesis. |
| + | |||
| + | Secondly, we want to get rid of the bacterium. Phages are natural predators of bacteria. They can also be used to transfect DNA into a bacterial cell. Phages has also the advantage of being specific to a strain and to be modulable. As we wanted to be eco-friendly, we create [[Team:Aix-Marseille/Bacteriophages|phage-like particles]], that aren't able to spread. Thus we have nanobots specific to [[Team:Aix-Marseille/Xylella_fastidiosa|''X. fastidiosa'']], capable to inject toxic genes into the bacterium. | ||
| + | |||
| + | The main cause of the plants death, is the hydric stress induced by the accumulation of biofilm into the xylem vessels. To disrupt the biofilm we thought about different solutions. The first one is to stop the bacterium producing any extra poly-saccharide. This could be achieved by [[Team:Aix-Marseille/QS|quenching the quorum sensing]] of the bacterium with the help of a little fatty acid called : 2-cis-decenoic acid. Secondly, we wanted to destroy the exo-polysaccharids. An [[Team:Aix-Marseille/DEPS|enzyme]] coming from a bacteriophage could fulfill the use by the hydrolysis of polysaccharides. | ||
| + | |||
| + | Hence, '''KILL XYLL''' simply detects, disrupt and kill [[Team:Aix-Marseille/Xylella_fastidiosa|''Xylella fastidiosa'']]. | ||
| − | |||
==Modelling== | ==Modelling== | ||
Revision as of 10:18, 28 September 2017
KILL XYL
Contents
Our project, KILL XYL will be a cure against the disease caused by the plant pathogen Xylella fastidiosa. This disease currently causes the loss of thousands of hectares of European crops and no extensive cure exists against it.
At Aix-Marseille University we thought about a solution that enclose many aspect of the cure. First, we wanted to improve the detection of the disease. To do so we use a NDVI camera that will help us to see if the plant do photosynthesis or not. If the plant is heavily affected by Xylella fastidiosa, it support a hydric stress that stop the photosynthesis.
Secondly, we want to get rid of the bacterium. Phages are natural predators of bacteria. They can also be used to transfect DNA into a bacterial cell. Phages has also the advantage of being specific to a strain and to be modulable. As we wanted to be eco-friendly, we create phage-like particles, that aren't able to spread. Thus we have nanobots specific to X. fastidiosa, capable to inject toxic genes into the bacterium.
The main cause of the plants death, is the hydric stress induced by the accumulation of biofilm into the xylem vessels. To disrupt the biofilm we thought about different solutions. The first one is to stop the bacterium producing any extra poly-saccharide. This could be achieved by quenching the quorum sensing of the bacterium with the help of a little fatty acid called : 2-cis-decenoic acid. Secondly, we wanted to destroy the exo-polysaccharids. An enzyme coming from a bacteriophage could fulfill the use by the hydrolysis of polysaccharides.
Hence, KILL XYLL simply detects, disrupt and kill Xylella fastidiosa.
Modelling
InterLab
We used plasmids containing different promoter from the 2017 distribution kit and transfect into E.coli. Before measuring fluorescence expression, we used LUDOX and fluorescein to calibrate the TECAN. We prepared a dilution series of fluorescein in 4 replicates and measure the fluorescence in a 96 well plate in a plate reader. By measuring these in all standard modes, we generated a standard curve of fluorescence for fluorescein concentration. We used this to correct cell based readings to an equivalent fluorescein concentration and convert this into a concentration of GFP.