(→Results) |
|||
| Line 5: | Line 5: | ||
[[File:T--Aix-Marseille--fluorondfort.jpeg|thumb|Gfp Fluorescence of '''E.coli''']] | [[File:T--Aix-Marseille--fluorondfort.jpeg|thumb|Gfp Fluorescence of '''E.coli''']] | ||
| − | In order to support the interlab approach, our team participate to the Fourth International InterLab Measurement Study. We are glad to contribute to this studie based on reliable and repeatable measurement. The challenge this year consist in etablishing a GFP measurement protocol that can be used produce comparable GFP measurements on plate readers. Furthermore, teams are also going to test some RBS devices that are intended to make gene expression more precise and reliable. | + | In order to support the interlab approach, our team participate to the Fourth International InterLab Measurement Study. We are glad to contribute to this studie based on reliable and repeatable measurement. The challenge this year consist in etablishing a GFP measurement protocol that can be used produce comparable GFP measurements on plate readers. This study aims to mesure fluorescence value from the E. coli K-12 DH5-alpha cells transformed with different plasmids, encoding for different promotor. Furthermore, teams are also going to test some RBS devices that are intended to make gene expression more precise and reliable. |
==Materials and Methods:== | ==Materials and Methods:== | ||
| Line 51: | Line 51: | ||
[[File:T--Aix-Marseille--fluo interlab.jpeg|right]] | [[File:T--Aix-Marseille--fluo interlab.jpeg|right]] | ||
| − | We used plasmids containing different promoter from the 2017 distribution kit and transfected them into | + | The InterLab Measurement Study has two components: calibration and cell measurement. The calibration component aims to identify the optimal setting for the plate reader to read the OD600 and fluorescence. We used plasmids containing different promoter from the 2017 distribution kit and transfected them into E.coli. |
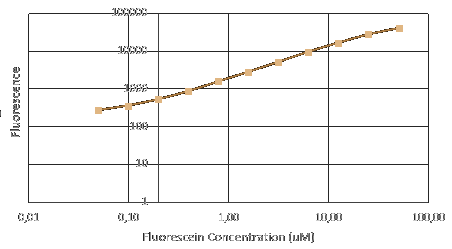
| − | + | Before measuring fluorescence expression, we used LUDOX and fluorescein to calibrate the TECAN. Also, we prepared a dilution series of fluorescein in 4 duplicates and measure the fluorescence in a 96 well plate in a plate reader. By measuring these in all standard modes, we generated a standard curve of fluorescence for fluorescein concentration. We used this to correct cell based readings to an equivalent fluorescein concentration and convert this into a concentration of GFP. | |
For more details on the procotol : | For more details on the procotol : | ||
| Line 79: | Line 79: | ||
In addition, we interessing in RBS devices (BCDs) that are intended to make gene expression more precise and reliable. Device 1, device 2 and device 3 contain rbs B0034. Device 4, device 5 and device 6 contain rbs BCD2. That can explain experimental results, regarding to theoretical strengh for promotors. For example, we observe a higher GFP signal for device 3 than device 5, despite of an stronger promotor for device 5. | In addition, we interessing in RBS devices (BCDs) that are intended to make gene expression more precise and reliable. Device 1, device 2 and device 3 contain rbs B0034. Device 4, device 5 and device 6 contain rbs BCD2. That can explain experimental results, regarding to theoretical strengh for promotors. For example, we observe a higher GFP signal for device 3 than device 5, despite of an stronger promotor for device 5. | ||
| − | |||
Promotor strength(iGEM datas): | Promotor strength(iGEM datas): | ||
* J23101(TD1,TD4):1791 | * J23101(TD1,TD4):1791 | ||
Revision as of 16:50, 26 October 2017
InterLab
How close can the numbers be when fluorescence is measured all around the world ?
In order to support the interlab approach, our team participate to the Fourth International InterLab Measurement Study. We are glad to contribute to this studie based on reliable and repeatable measurement. The challenge this year consist in etablishing a GFP measurement protocol that can be used produce comparable GFP measurements on plate readers. This study aims to mesure fluorescence value from the E. coli K-12 DH5-alpha cells transformed with different plasmids, encoding for different promotor. Furthermore, teams are also going to test some RBS devices that are intended to make gene expression more precise and reliable.
Materials and Methods:
The InterLab Measurement Study has two components: calibration and cell measurement. The calibration component aims to identify the optimal setting for the plate reader to read the OD600 and fluorescence. We used plasmids containing different promoter from the 2017 distribution kit and transfected them into E.coli.
Before measuring fluorescence expression, we used LUDOX and fluorescein to calibrate the TECAN. Also, we prepared a dilution series of fluorescein in 4 duplicates and measure the fluorescence in a 96 well plate in a plate reader. By measuring these in all standard modes, we generated a standard curve of fluorescence for fluorescein concentration. We used this to correct cell based readings to an equivalent fluorescein concentration and convert this into a concentration of GFP.
For more details on the procotol : iGEM Protocol
Results
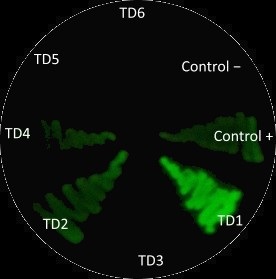
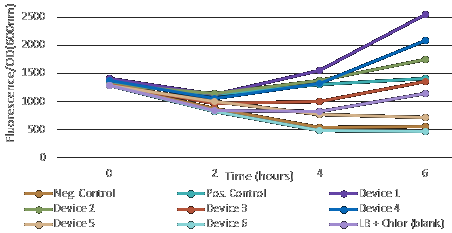
After measuring fluorescence readings and OD, we calculed ratio of Fluorescence/OD in order to determine promotor strengh. Device 1 and device 4 containing promoter J23101 had high fluorescence production. Device 2 (J23106) and device 3 (J23117) was observed to have a media increase of fluorescence production.On contrary, device 5 (J23106) and device 6 (J23117)maked a low fluorescence production.
This results are suggesting that the promoter J23101 had the strongest affinity for initial RNA polymerase binding comparing to other devices promoter J23117 and J23106.
This results lead to discuss about GFP expression by different constructions.
In addition, we interessing in RBS devices (BCDs) that are intended to make gene expression more precise and reliable. Device 1, device 2 and device 3 contain rbs B0034. Device 4, device 5 and device 6 contain rbs BCD2. That can explain experimental results, regarding to theoretical strengh for promotors. For example, we observe a higher GFP signal for device 3 than device 5, despite of an stronger promotor for device 5.
Promotor strength(iGEM datas):
- J23101(TD1,TD4):1791
- J23106(TD2,TD5):1185
- J23117(TD3,TD6):162
In all, we can supposed that device 1 and 4 have the strongest promotor. The point on RBS is that B0034 owns an higher specificity, explaining why device 1 is higher thant device 4.