| Line 7: | Line 7: | ||
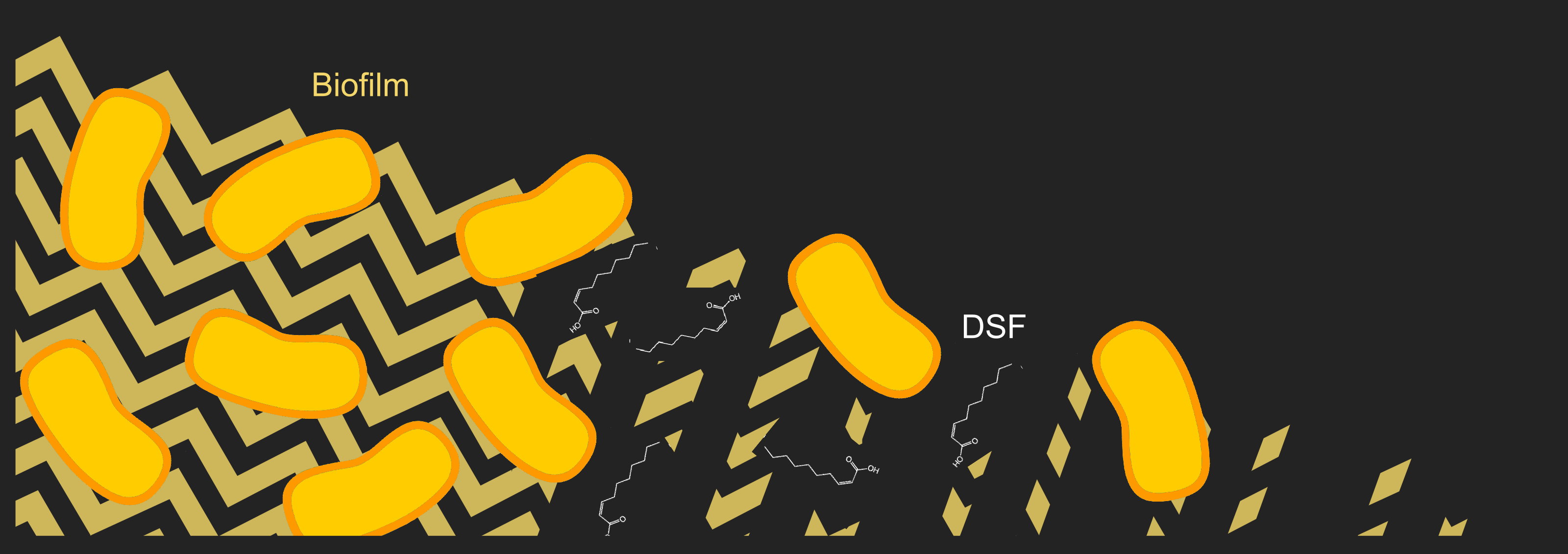
[[File:T--Aix-Marseille--QSexp.png|500px|right|thumb| Utilization of the DSF 2-cis-decenoic acid to stop the biofilm formation of bacteria.]] | [[File:T--Aix-Marseille--QSexp.png|500px|right|thumb| Utilization of the DSF 2-cis-decenoic acid to stop the biofilm formation of bacteria.]] | ||
| − | In order to produce this biofilm, to communicate and coordinate the gene expression of the whole colony, [[Team:Aix-Marseille/Xylella_fastidiosa|''X. fastidiosa'']] mainly uses a Diffusible Signal Factor (DSF) which is a fatty acid with a 2-cis unsaturation<ref>Ionescu, M. et al. Promiscuous Diffusible Signal Factor Production and Responsiveness of the Xylella fastidiosa Rpf System. mBio 7, e01054–16 (2016).</ref>. | + | In order to produce this biofilm, to communicate and coordinate the gene expression of the whole colony, [[Team:Aix-Marseille/Xylella_fastidiosa|''X. fastidiosa'']] mainly uses a Diffusible Signal Factor (DSF) which is a fatty acid with a 2-cis unsaturation<ref>Ionescu, M. et al. Promiscuous Diffusible Signal Factor Production and Responsiveness of the ''Xylella fastidiosa'' Rpf System. mBio 7, e01054–16 (2016).</ref>. |
The same system of communication regulates virulence. | The same system of communication regulates virulence. | ||
In addition to quorum sensing, bacteria can also use a similar mechanism called quorum quenching to modify the quorum sensing of other bacterial populations and outcompete them. | In addition to quorum sensing, bacteria can also use a similar mechanism called quorum quenching to modify the quorum sensing of other bacterial populations and outcompete them. | ||
Revision as of 02:14, 2 November 2017
Quorum sensing
Contents
Quorum sensing is a mechanism that allows bacteria to coordinate their behaviour depending on the bacterial population density [1]. It allows bacteria to adopt collective patterns of gene regulation to have, at a population level, an advantageous phenotype. Quorum sensing allows Xylella fastidiosa to produce a biofilm, a sticky extracellular matrix composed of DNA, proteins and polysaccharides, which is one of the key problems in the plant disease because it blocks xylem vessels.
In order to produce this biofilm, to communicate and coordinate the gene expression of the whole colony, X. fastidiosa mainly uses a Diffusible Signal Factor (DSF) which is a fatty acid with a 2-cis unsaturation[2]. The same system of communication regulates virulence. In addition to quorum sensing, bacteria can also use a similar mechanism called quorum quenching to modify the quorum sensing of other bacterial populations and outcompete them. Therefore, to stop X. fastidiosa forming the biofilm that kills plants we choose to quench it quorum sensing activity.
Pseudomonas aeruginosa under stress produces a similar fatty acid named 2-cis-decenoic acid, that is able to stop biofilm formation by many organisms[3]. Thus, we thought of including this specific DSF in KILL XYL to stop X. fastidiosa biofilm formation.
Design
As we wanted to limit the number of GMOs in our product we wanted to produce and purify the 2-cis-decenoic acid in E. coli. We choose to not use P. aeruginosa because of it pathogenicity.
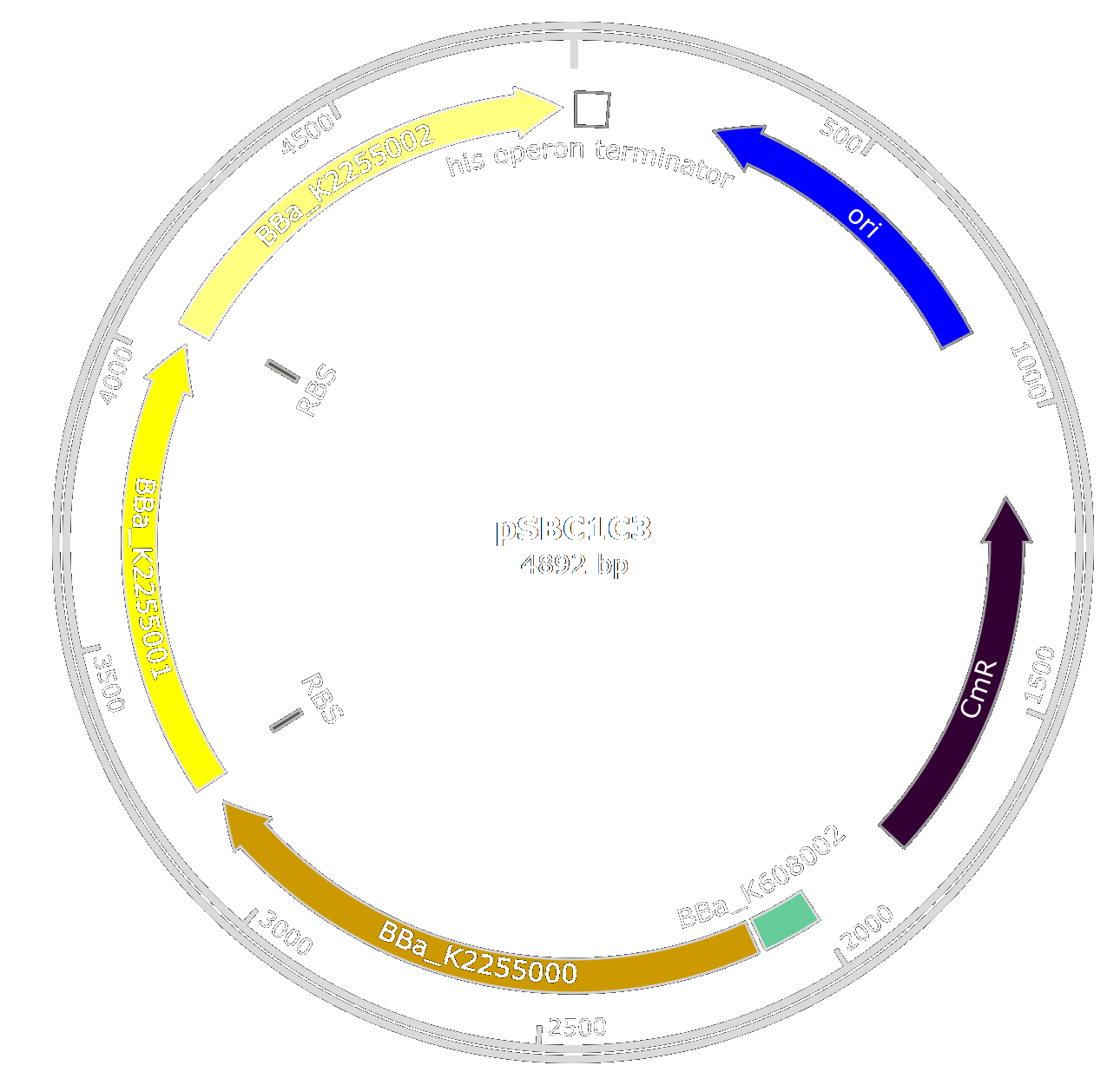
Several enzymes are necessary for the production of 2-cis-decenoic acid by Pseudomonas aeruginosa, thay are not all present in E. coli. Thus, to produce the fatty acid, we designed biobricks to produce the three enzymes lacking in E. coli for the production of 2-cis-decenenoic and we optimized their sequences for production in E. coli. Part [http://parts.igem.org/Part:BBa_K2255000 BBa_K2255000] is an enoyl-CoA hydratase, [http://parts.igem.org/Part:BBa_K2255001 BBa_K2255001] an acyl-CoA isomerase and [http://parts.igem.org/Part:BBa_K2255002 BBa_K2255002] a thioesterase.
We want to optimize the production of the different proteins so we designed these parts so they can be assembled to form an operon. Thus, [http://parts.igem.org/Part:BBa_K2255001 BBa_K2255001] and [http://parts.igem.org/Part:BBa_K2255002 BBa_K2255002] have a Ribosome Binding Site (RBS) integrated into the biobrick. To produce large amounts of these enzymes, we decided to add a strong and constitutive promoter in E.coli ([http://parts.igem.org/Part:BBa_K608002 BBa_K608002]).
Results
As a proof of concept, we studied the effect of the fatty acid on X. campestris. We used several different experimental conditions, such as the concentration of product and type of tube. After growing bacteria in media containing the product, we measured the effect of it on biofilm production using crystal violet visually (Protocol). Biofilm production can also be quantified with TECAN and is proportional with absorbance. We measured fatty acid effect by adding it at the start of growth. Then we observed biofilm production after 24, 48, 72, and 96 hours.
These results are very encouraging. We can see a significant decrease of biofilm production after 48, 72 and 96 hours. However, they need to be reproduced and the biofilm quantified and studied in more detail.
References
- ↑ Rutherford, S. T. & Bassler, B. L. Bacterial Quorum Sensing: Its Role in Virulence and Possibilities for Its Control. Cold Spring Harb Perspect Med 2, a012427 (2012).
- ↑ Ionescu, M. et al. Promiscuous Diffusible Signal Factor Production and Responsiveness of the Xylella fastidiosa Rpf System. mBio 7, e01054–16 (2016).
- ↑ Amari, D. T., Marques, C. N. H. & Davies, D. G. The Putative Enoyl-Coenzyme A Hydratase DspI Is Required for Production of the Pseudomonas aeruginosa Biofilm Dispersion Autoinducer cis-2-Decenoic Acid. J. Bacteriol. 195, 4600–4610 (2013).