Cccxyyyyyyyy (Talk | contribs) |
|||
| Line 368: | Line 368: | ||
</p> | </p> | ||
| − | <p><i>Vika-vox</i> system mainly consists of <i>vox</i> sites and reporting parts. At first the expression of <i> | + | <p><i>Vika-vox</i> system mainly consists of <i>vox</i> sites and reporting parts. At first the expression of <i>yEmRFP</i> will be activating and the expression of <i>β-carotene</i> will be inhibited so that we can detect red fluorescence when recombinase <i>vika</i> doesn’t exist in <i>Saccharomyces cerevisiae</i>. After the expression of recombinase <i>vika</i>, with the deletion of <i>yEmRFP</i> and terminators flanked by <i>vox</i> locus, <i>β-carotene</i> expresses and the strains take on an orange color. This is the whole characterization process of Mating Switcher. |
</p> | </p> | ||
| Line 401: | Line 401: | ||
<hr> | <hr> | ||
<h5>1) Construction of <i>vox-ura3-terminator-vox</i> Structure </h5> | <h5>1) Construction of <i>vox-ura3-terminator-vox</i> Structure </h5> | ||
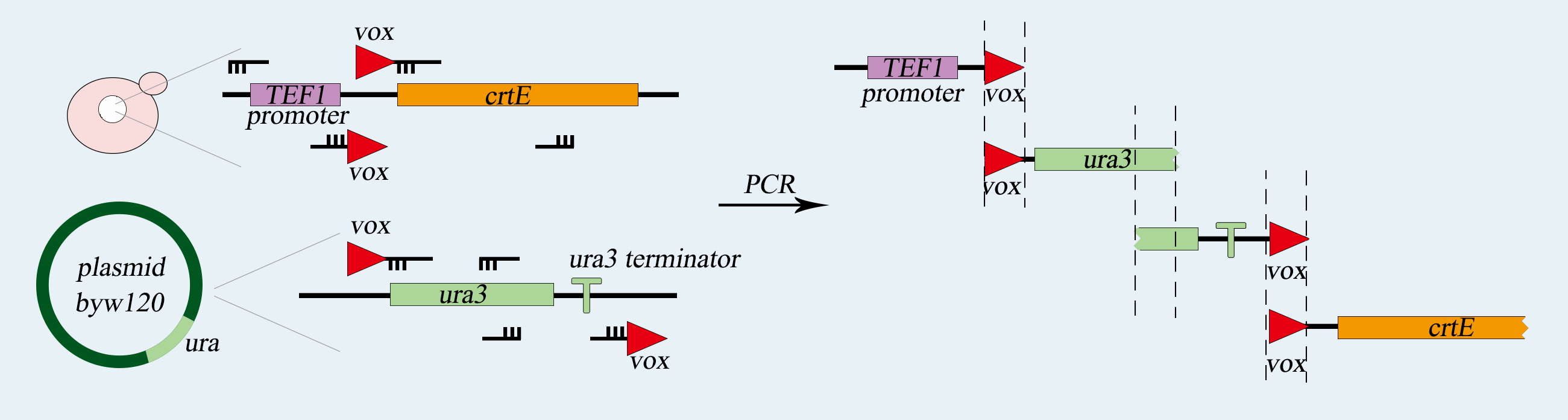
| − | <p> We use <i> | + | <p> We will use <i>Syn.Ⅴ</i> of <i>Saccharomyces cerevisiae</i> to load our device, which is a haploid with mating type of a. First of all, we use PCR to amplify basic parts including <i>TEF</i> promoter, <i>ura3</i> gene, <i>ura3-terminator</i> and <i>β-carotene</i> gene. Among them, <i>ura3</i> gene and <i>ura3-terminator</i> are flanked by <i>vox</i> locus. Then we use overlap PCR to combine these parts together. The next step is transform this composite part into <i>Saccharomyces cerevisiae</i>. We screen for the correctly transformed cell by using the <i>Sc-Ura</i> plate. For the purpose of verifying desired strain <i><b>PVUVC</b></i>, we use colony PCR to amplify the <i>TEF promoter-vox-ura3</i> structure and <i>ura3 terminator-vox-β-carotene</i> structure. The length of the strip will be observed by agarose gel electrophoresis. |
</p> | </p> | ||
| Line 419: | Line 419: | ||
<h5>2) Construction of <i>vox-RFP-terminators-vox</i> Structure</h5> | <h5>2) Construction of <i>vox-RFP-terminators-vox</i> Structure</h5> | ||
| − | <p> This structure has a great similarity to the <i>vox-ura3-terminator-vox</i> structure above. Therefore, it is easy to construct because we only need to change the <i>ura3</i> gene to the | + | <p> This structure has a great similarity to the <i>vox-ura3-terminator-vox</i> structure above. Therefore, it is easy to construct because we only need to change the <i>ura3</i> gene to the <i>yEmRFP</i> gene. We use PCR to amplify five basic prats including <i>TEF promoter</i>, <i>yEmRFP gene</i>, <i>Adh1 terminator</i>, <i>ura3-terminator</i> and <i>β-carotene</i> gene. Among them, <i>yEmRFP</i> gene and <i>ura3-terminator</i> are flanked by <i>vox</i> locus. Then we use overlap PCR to combine these parts together. After that we use the lithium acetate conversion method to transfer this composite part into <i><b>TVUVC</b></i>. We screen for the correctly transformed cell by using the 5-FOA plate. This part will integrate into <i>Syn.Ⅴ</i> by homologous recombination, and we will get another desired strain called <i><b>TVRVC</b></i>. |
</p> | </p> | ||
| Line 436: | Line 436: | ||
| − | <h5>3) Verification of <i> | + | <h5>3) Verification of <i>yEmRFP</i> in the PVRVC</h5> |
| − | <p> The verification of <i> | + | <p> The verification of <i>yEmRFP</i> is carried out by using colony PCR to amplify the homologous arm-TEF promoter-vox-RFP gene and terminator-vox-crtE gene, which determine the existence of <i>vox</i> sites and <i>yEmRFP</i> gene. Then we can detect the red fluorescence. |
</p> | </p> | ||
| Line 445: | Line 445: | ||
</p> | </p> | ||
<h5>5) Construction of <i>Vika</i> System</h5> | <h5>5) Construction of <i>Vika</i> System</h5> | ||
| − | <p>We use a common expression vector plasmid, <i>pRS416</i>, to load <i>vika</i> part. First of all, we use corresponding restriction endonuclease <i>Sal1</i> and <i>Not1</i> to cut plasmid <i>pRS416</i> and plasmid <i>pRS415-vika</i>, a gift from Y.J lab, and then use <i>T4</i> DNA ligase to link them together, we can obtain the complete device we want. Finally, we transform this device into <i>BY4742</i> by the lithium acetate conversion method, and we screen for the correctly transformed cell by using the <i>Sc-Ura</i> plate. <i>BY4742</i> is a | + | <p>We use a common expression vector plasmid, <i>pRS416</i>, to load <i>vika</i> part. First of all, we use corresponding restriction endonuclease <i>Sal1</i> and <i>Not1</i> to cut plasmid <i>pRS416</i> and plasmid <i>pRS415-vika</i>, a gift from Y.J lab, and then use <i>T4</i> DNA ligase to link them together, we can obtain the complete device we want. Finally, we transform this device into <i>BY4742</i> by the lithium acetate conversion method, and we screen for the correctly transformed cell by using the <i>Sc-Ura</i> plate. <i>BY4742</i> is a haploid with mating type of <i>α</i>. |
</p> | </p> | ||
| − | <p> We also use another plasmid <i>pRS413</i> | + | <p> We also use another plasmid <i>pRS413</i> with previous method. |
</p> | </p> | ||
<h5>6) The Induction of Mating</h5> | <h5>6) The Induction of Mating</h5> | ||
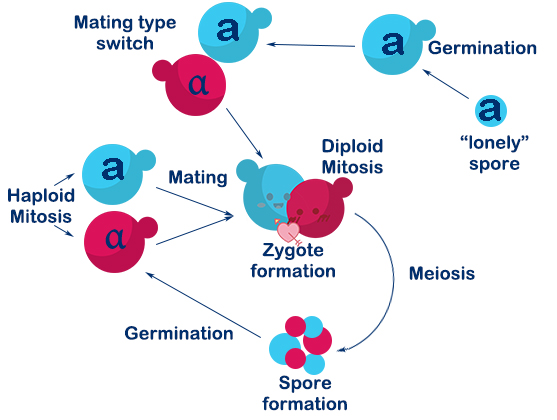
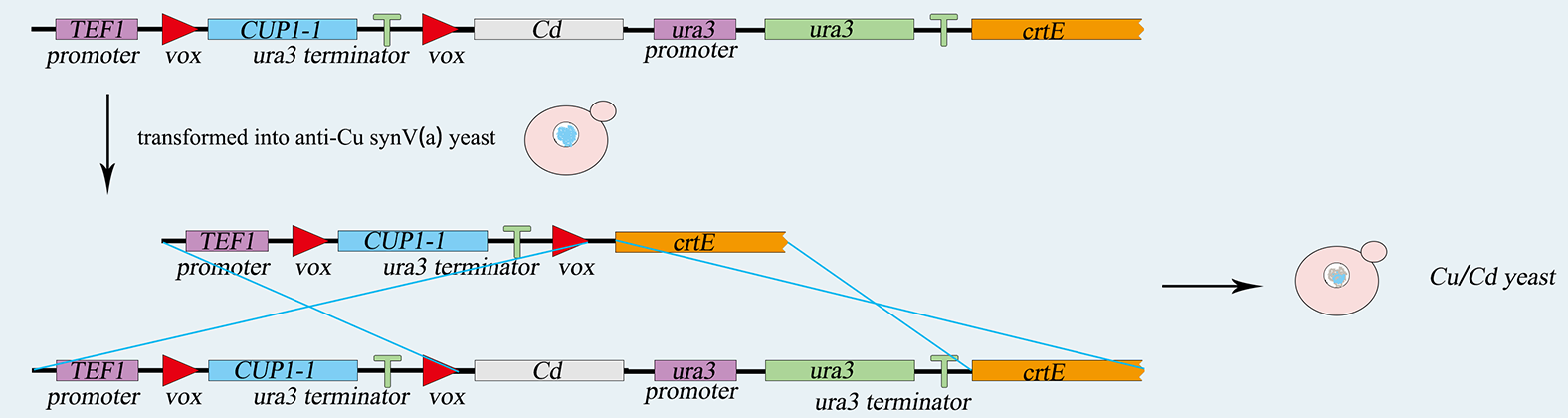
| − | <p> The <i>Saccharomyces cerevisiae</i> called <i><b>PVRVC</b></i> is a | + | <p> The <i>Saccharomyces cerevisiae</i> called <i><b>PVRVC</b></i> is haploid with mating type of <i>a</i>. At first, we cultivate <i>pRS416-vika</i> in <i>Sc-Ura</i> medium without glucose for three hours. To induce the expression of <i>vika</i>, they will be cultured to saturation in <i>Sc</i> medium with <i>raffinose</i> and <i>galactose</i> for twelve hours. After that, recombinase <i>vika</i> are induced to express and we make <i>α-pRS416-vika</i> cell and a-TVRVC cell mate in <i>YPD</i> medium for eight hours. Two types cells are fused and form diploid yeasts, in which recombinase <i>vika</i> bind with <i>vox</i> locus, and then delete <i>yEmRFP</i> gene and <i>Adh1</i> terminator flanked by <i>vox</i> sites. After the Mating Switcher, <i>β-carotene</i> expresses and the color of cell will transform from white to orange. At last we smear yeast solution on <i>Sc-Ura-Leu</i> plate to screen for the correctly mating cell. We can judge the existence of recombinase <i>vika</i> by the color of the colony, and obtain the efficiency of mating. |
</p> | </p> | ||
| Line 467: | Line 467: | ||
</p> | </p> | ||
| − | <p> Second, we expect to observe the expression of | + | <p> Second, we expect to observe the expression of <i>yEmRFP</i> before the switch is on. We expect red fluorescent protein to be observed under a fluorescence microscope, and it is desirable that the fluorescence spectrophotometer can measure a high and stable fluorescence value. |
</p> | </p> | ||
| − | <p> Third, <i>vika-vox</i> system can play a role on function conversion. It is means that β-carotene gene can express after mating. We will observe | + | <p> Third, <i>vika-vox</i> system can play a role on function conversion. It is means that β-carotene gene can express after mating. We will observe orange colonies on nutrient label medium. |
</p> | </p> | ||
Revision as of 14:48, 1 November 2017
/* OVERRIDE IGEM SETTINGS */
Design
Background
Human existence on earth is almost impossible without the heavy metals. Even though important to mankind, exposure to them during production, usage and their uncontrolled discharge into the environment has caused lots of hazards to man, other organisms and the environment itself. Heavy metals can enter human tissues and organs via inhalation, diet, and manual handling. As the process of urbanization and industrialization goes deeper and deeper, heavy metal pollution, a noticeable threaten to almost all the creatures, has become an essential problem to solve.
According to our human practice, the situation of heavy metal pollution (copper and cadmium ions) is marked on a world map, and the severity of heavy metal pollution has been increasing all over this map. Places with serious pollution include middle Asia, eastern Asia, southern Europe, and Latin America. In addition, not only fresh water source, but also soil and crops are seriously contaminated by heavy metals. On average, during three out of ten suppers we have, we absorb excess heavy metals over the standard concentration.
Considering the rigorous situation we face, our team decided to design an advanced system for typical toxic heavy metal disposal based on Saccharomyces cerevisiae.