Erwan Eriau (Talk | contribs) m |
Erwan Eriau (Talk | contribs) (fig1) |
||
| Line 34: | Line 34: | ||
'''Results''' | '''Results''' | ||
| + | |||
| + | [[File:|400px|right|thumb|Differences in affinity of host's DNA polymerase III ("Polymerase Binding Event Helper" and "Polymerase Binding Event Phagemid" in the model) for the E-coli origin of replication impacts the ratio of produced PLPs / replicative phage. These parameters are determined by the backbone we choose for our constructs.]] | ||
We need one or two graphs here showing how the packaging ratio depends on three main parameters: the DNA III polymerase binding rate to the E-coli origin of replication (Figure 2) of either the plasmid or phagemid, the initial ratio of transfected phagemid and helper phage (Figure 3), and the difference in p5 affinity for eitheir plasmid's M13 ori (Figure 4). | We need one or two graphs here showing how the packaging ratio depends on three main parameters: the DNA III polymerase binding rate to the E-coli origin of replication (Figure 2) of either the plasmid or phagemid, the initial ratio of transfected phagemid and helper phage (Figure 3), and the difference in p5 affinity for eitheir plasmid's M13 ori (Figure 4). | ||
Revision as of 14:39, 1 November 2017
Modelling PLP production
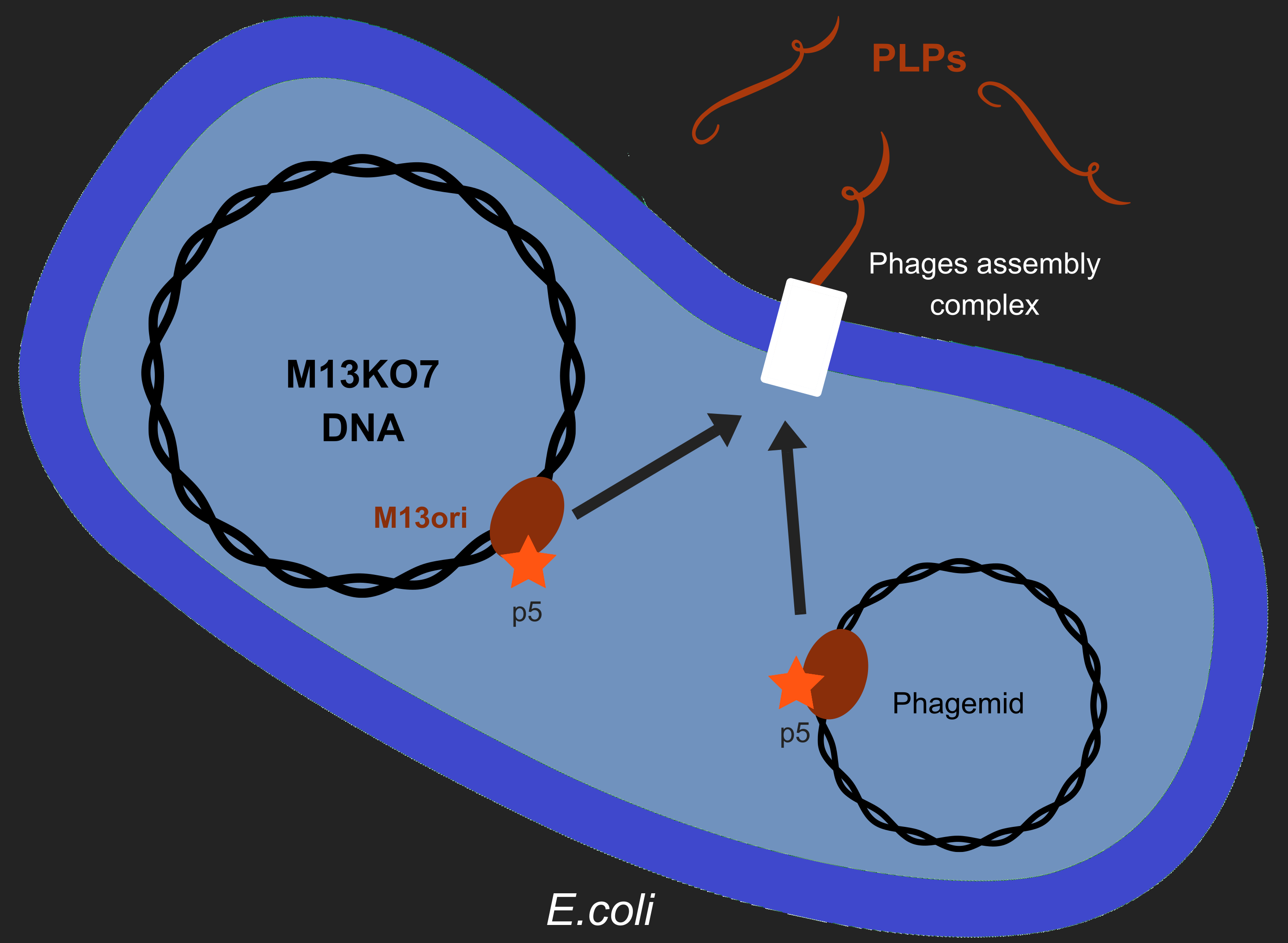
The problem
Our objective is to produce phage like particles (PLP), for this, the bacteria must contain both a helper phage and also a phagemid (Figure 1). During phage production, several key events determine which DNA molecules are packaged into phage or PLP. These involve recognition of the M13 replication origin by several phage proteins.
A major hurdle to marketing KILL XYL is obtaining the necessary authorizations. Our interviews and legislation study both showed that the number of viable phages could be a problem. We therefore decided to measure and model the ratio between viable phage and phage-like particles, and so try to optimize this ratio to facilitate the preparation of pure PLP.
The Model
We based our model on a recently published model of "wild-type" M13 replication [1] [2]. This model was modified to incorporate: The helper-phage E. coli plasmid origin; the modification of the helper-phage M13 origin; the presence of a phagemid, with its own replicative origin and M13 origin. These modifications added xxx species, zzz equations and yyy parameters to the original model, and they modified www parameters of the original model. The modified model is available File:T--Aix-Marseille--Model.zip .
We used the initial measurement to constrain parameters of the model, along with published numbers for the number of copies of plasmids with different replicative origins.
Results
[[File:|400px|right|thumb|Differences in affinity of host's DNA polymerase III ("Polymerase Binding Event Helper" and "Polymerase Binding Event Phagemid" in the model) for the E-coli origin of replication impacts the ratio of produced PLPs / replicative phage. These parameters are determined by the backbone we choose for our constructs.]]
We need one or two graphs here showing how the packaging ratio depends on three main parameters: the DNA III polymerase binding rate to the E-coli origin of replication (Figure 2) of either the plasmid or phagemid, the initial ratio of transfected phagemid and helper phage (Figure 3), and the difference in p5 affinity for eitheir plasmid's M13 ori (Figure 4). We also observed increasing the number of transfected plasmids increased production, up to a certain point, which we determined (Figure 5).
A description of these graphs.
Conclusions
Clearly the initial design needs to be improved to increase the proportion of PLP produced.
The model has shown as that the parameters of prime importance for determining the proportion of PLP produced are .... .- ↑ Smeal et al, Simulation of the M13 life cycle I: Assembly of a genetically-structured deterministic chemical kinetic simulation, Virology, 500, January 2017
- ↑ Smeal et al, Simulation of the M13 life cycle II: Investigation of the control mechanisms of M13 infection and establishment of the carrier state, Virology, 500, January 2017

