KILL XYL
Contents
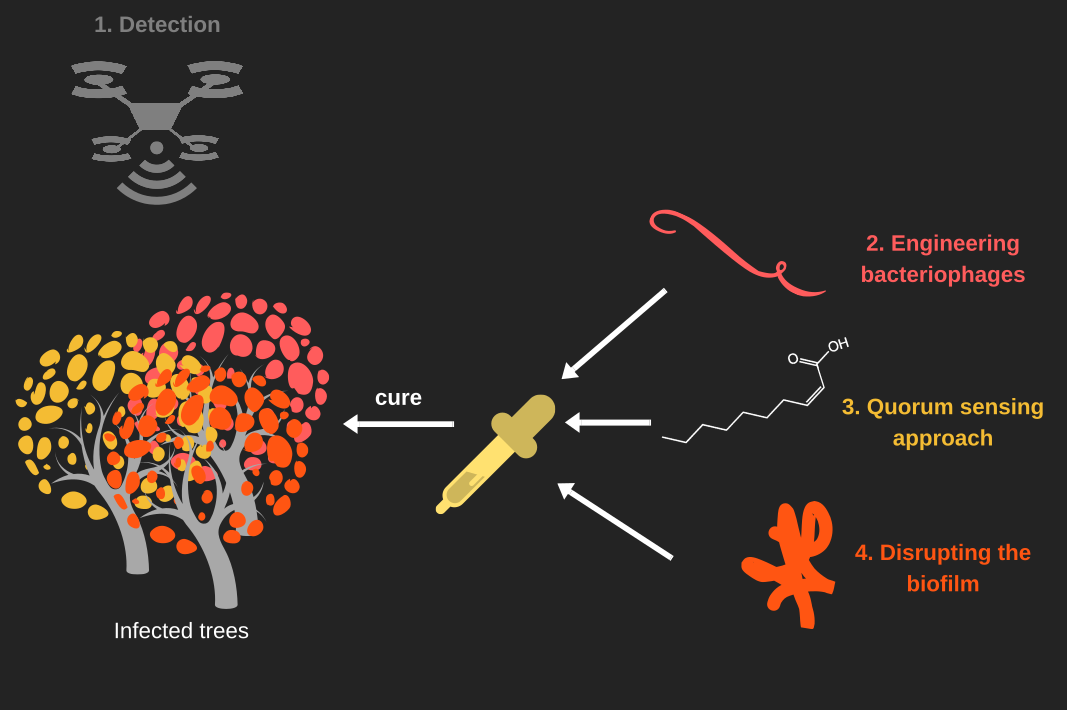
At Aix-Marseille University we thought about a solution that enclose many aspect of the cure. First, we wanted to improve the detection of the disease, to do so we wanted to use a NDVI camera that will help us to see if the plant do photosynthesis or not. If the plant is heavily affected by Xylella fastidiosa, it support a hydric stress that stop the photosynthesis.
Secondly, we want to get rid of the bacterium. Phages are natural predators of bacteria. They can also be used to transfect DNA into a bacterial cell. Phages has also the advantage of being specific to a strain and to be modulable. As we wanted to be eco-friendly, we create phage-likes particles, that aren't able to spread. Thus we have nanobots specific to X. fastidiosa, capable to inject toxic genes into the bacterium.
The main cause of the plants death, is the hydric stress induced by the accumulation of biofilm into the xylem vessels. To disrupt the biofilm we thought about different solutions. The first one is to stop the bacterium producing any extra poly-saccharide. This could be achieved by quenching the quorum sensing of the bacterium with the help of a little fatty acid called : 2-cis-decenoic acid. Secondly, we wanted to destroy the exo-polysaccharids. An enzyme coming from a bacteriophage could fulfill the use by the hydrolysis of polysaccharides.
Hence, KILL XYLL simply detects, disrupt and kill Xylella fastidiosa.
Detection of the disease
The purpose of the detection would be to determine if the tree is infected by X. fastidiosa For this, there is a method called PCR (Polymerase Chain Reaction) that can detect the presence of viruses or measure viral loads. However, this is a complex method that requires DNA sampling on the tree, which will undergo various treatments in the laboratory, so it is not a way to detect X. fastidiosa on the field.
Therefore, this project focuses on detecting the drying of the tree which is one of the first symptoms of X. fastidiosa. In the case of the olive tree, this method is relevant because the olive tree is an evergreen tree, its leaves will not dry naturally if the tree is healthy. However, the drying of the tree can be caused by other factors than X. fastidiosa, the solution presented here serves primarily as a warning device, it is appropriate after detecting the drying of a tree to take a sample and to ensure that it is X. fastidiosa which infects the tree before injecting the remedy developed by the team.
Read more about our detection hardware...
Engineering bacteriophages
Bacteriophages play a special role in nanoscale cargo-delivery developments, because they can be regarded as naturally occurring nanomaterials. We choose to explore the VNPs use against pathogenic bacterium like X. fastidiosa. Our goal i to use a phage, like M13, in order to inject a lethal toxin into the bacterium.
Quorum sensing approach
X. fastidiosa uses quorum sensing, as an inter-bacterial communication system, to regulate its biofilm production. The quorum sensing is based on the emission of specific fatty acid, there are fatty acids antagonistic to its quorum sensing, this disturbance is called quorum quenching. 2-cis-decenoic acid is one of these fatty acids whom has quorum quenching effect to X. fastidiosa. This action of quenching will prevent the formation of biofilm, dependent of the quorum sensing and therefore will have both action preventive and curative to the symptoms caused by the biofilm on the plants. We want to produce this fatty acids from E. coli to inoculated the infected trees. Thus saving the plants from X. fastidiosa.
Disrupting the biofilm
Extracellular polysaccharide (EPS) is a major virulence factor of X. fastidiosa, the causative agent of Olive Quick Decline (OQD) associated with binding of water, ions and nutrients; keeping them in close contact with the bacteria; and protection against the recognition by plant cell defense mechanisms. Some bacteriophages carry coat proteins that can degrade bacterial polysaccharides. The Depolymerase binds to the capsular EPS and degrades the polymer until the phage reaches the cell surface, where it binds to an outer membrane receptor and injects nucleic acid to initiate the lytic cycle. To disrupt the biofilm we centered our research on this enzyme, which we called deps to cleave the extracellular polysaccharides of X. fastidiosa freeing up the tension cause by EPS in the xylem vessels. I hope that curing the plant from the hydric stress caused by the bacteria.