X

Project

Experiments
Modeling

Prototype

Human Practices

Safety

About Us

Attributions
Project
Experiment
Modeling
Prototype
Human Practice
Biosafety
About Us
Attributions

hi
PROTOTYPE
It is estimated that about 95% of nanoparticles used in consumer products end up in wastewater (Kiser et al. 2009). Our goal is to apply our biofilm and Proteorhodopsin (PR) bacteria in wastewater treatment plants (WWTPs) to remove most nanoparticles (NPs) before the effluent is released into the environment.
WASTEWATER TREATMENT
What is the process?

When wastewater enters a plant, the first step is to remove coarse solids and large materials using a grit screen (figure___). The water can then be processed in three main stages: Primary, Secondary, and sometimes Tertiary Treatment (Pescod 1992). In Primary Treatment, heavy solids are removed by sedimentation, and floating materials (such as oils) can be taken out by skimming. However, dissolved materials and colloids—small, evenly dispersed solids such as nanoparticles—are not removed here (Pescod 1992). Secondary Treatment generally involves the use of aeration tanks, where aerobic microbes help to break down organic materials. This is also known as the activated sludge process (Davis 2005). In a subsequent sedimentation step, the microbes are removed and the effluent is disinfected (often by chlorine or UV) before it is released into the environment. In certain WWTPs, wastewater may go through Tertiary Treatment, an advanced process typically aimed to remove nitrogen and phosphorous, and assumed to produce an effluent free of viruses. However, Tertiary Treatment requires additional infrastructure that is expensive and complex, limiting its global usage (Pescod 1992; Malik 2014).
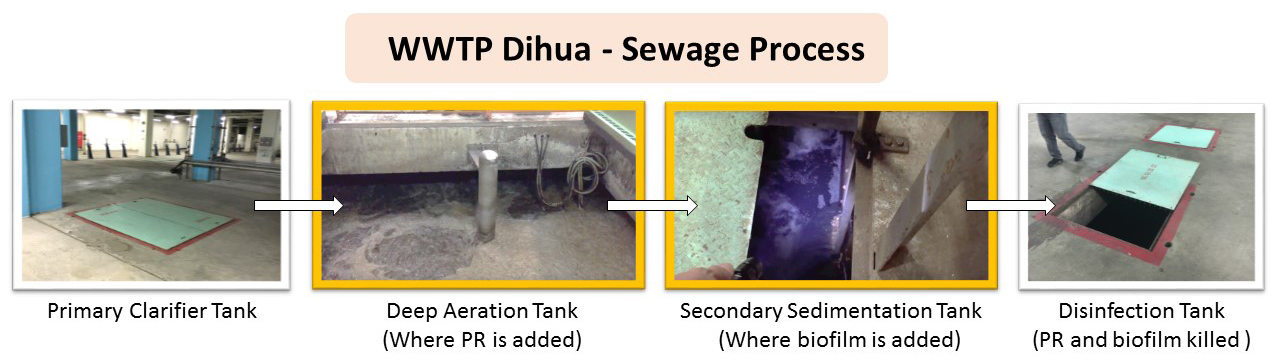
We plan to add our bacteria either in the deep aeration tanks or the secondary sedimentation tanks. The disinfection tank will kill the bacteria used in previous tanks.Figure: Christine C.

We plan to add our bacteria either in the deep aeration tanks or the secondary sedimentation tanks. The disinfection tank will kill the bacteria used in previous tanks.Figure: Christine C.
Biosafety
We have chosen to use a safe and common lab strain of E. coli, K-12, as our chassis (Environmental Protection Agency 1977). In both approaches, our constructs do not express proteins associated with virulence: PR is a membrane protein that commonly exists in marine bacteria, and for biofilm production we were careful to avoid known virulence factors such as alpha hemolysins (Fattahi et al. 2015). Most importantly, biosafety is built into WWTPs. Before treated effluent is released back into the environment, it must go through a final disinfection step, where chlorine, ozone, or UV radiation are used to kill microbes still present in the wastewater (Pescod 1992).
APPLYING PR IN WWTPs
To achieve our goal of applying biofilms in WWTPs, we need to inform WWTP managers on the amount of biofilm necessary to trap their desired amount of NPs. Thus, we devised two experiments to investigate the effect of 1) biofilm volume and 2) biofilm surface area on NP trapping; the results of these experiments were incorporated into our model. (Learn more about modeling here!)
APPLYING BIOFILM IN WWTPs
Volume Does Not Affect NP Trapping
To test the effects of biofilm volume, E. coli biofilms were grown, extracted, and washed as described in the Experimental page. Then, 10 mL of Gold NP (AuNP) solution was added to different volumes of biofilm (figure ______). The containers were shaken at 100 rpm overnight to maximize interaction between the biofilm and AuNPs. Because AuNP solution is purple in color, we could take absorbance measurements and convert these values to AuNP concentration using a standard curve (figure ______). Finally, the mixtures were transferred to conical tubes and centrifuged to isolate the supernatant, which contains free AuNPs quantifiable using a spectrophotometer set at 527 nm.



