Quorum sensing
Contents
Quorum sensing is a mechanism that allows bacteria to coordinate their behaviour depending on the bacterial population density [1]. It allows bacteria to adopt collective patterns of gene regulation to have, at a population level, an advantageous phenotype. Quorum sensing allows Xylella fastidiosa to produce a biofilm, a sticky extracellular matrix composed of DNA, proteins and polysaccharides, which is one of the key problems in the plant disease because it blocks xylem vessels.
In order to produce this biofilm, to communicate and coordinate the gene expression of the whole colony, X. fastidiosa mainly uses a Diffusible Signal Factor (DSF) which is a fatty acid with a 2-cis unsaturation[2]. The same system of communication regulates virulence. In addition to quorum sensing, bacteria can also use a similar mechanism called quorum quenching to modify the quorum sensing of other bacterial populations and outcompete them. Therefore, to stop X. fastidiosa forming the biofilm that kills plants we choose to quench it quorum sensing activity.
Pseudomonas aeruginosa under stress produces a similar fatty acid named 2-cis-decenoic acid, that is able to stop biofilm formation by many organisms[3]. Thus, we thought of including this specific DSF in KILL XYL to stop X. fastidiosa biofilm formation.
Design
As we wanted to limit the number of GMOs in our product we wanted to produce and purify the 2-cis-decenoic acid in E. coli. We choose to not use P. aeruginosa because of it pathogenicity.
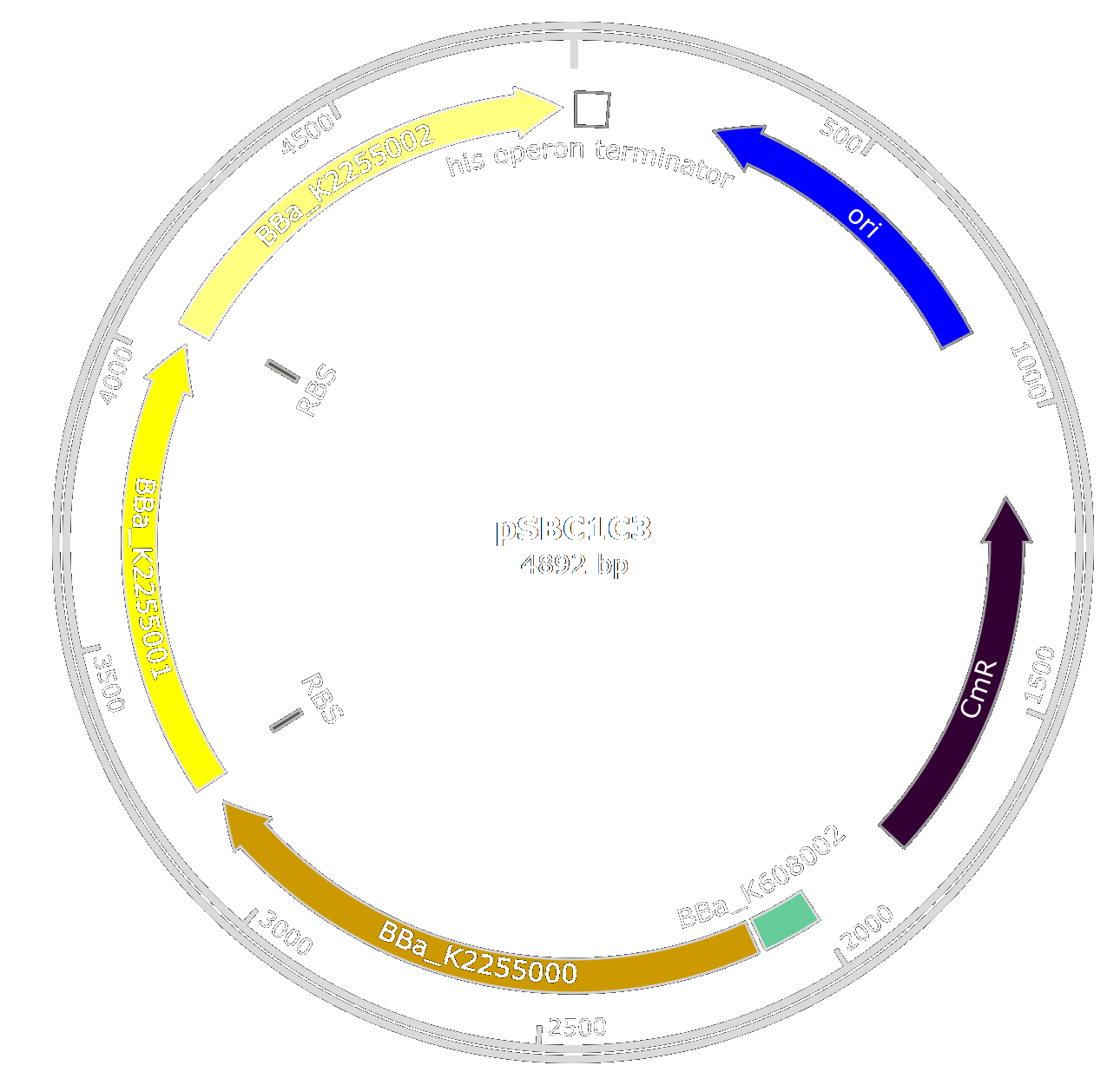
Several enzymes are necessary for the production of 2-cis-decenoic acid by Pseudomonas aeruginosa, thay are not all present in E. coli. Thus, to produce the fatty acid, we designed biobricks to produce the three enzymes lacking in E. coli for the production of 2-cis-decenenoic and we optimized their sequences for production in E. coli. Part [http://parts.igem.org/Part:BBa_K2255000 BBa_K2255000] is an enoyl-CoA hydratase, [http://parts.igem.org/Part:BBa_K2255001 BBa_K2255001] an acyl-CoA isomerase and [http://parts.igem.org/Part:BBa_K2255002 BBa_K2255002] a thioesterase.
We want to optimize the production of the different proteins so we designed these parts so they can be assembled to form an operon. Thus, [http://parts.igem.org/Part:BBa_K2255001 BBa_K2255001] and [http://parts.igem.org/Part:BBa_K2255002 BBa_K2255002] have a Ribosome Binding Site (RBS) integrated into the biobrick. To produce large amounts of these enzymes, we decided to add a strong and constitutive promoter in E.coli ([http://parts.igem.org/Part:BBa_K608002 BBa_K608002]).
Results
As a proof of concept, we studied the effect of the fatty acid on X. campestris. We used several differents experimental conditions, such as concentration of product and type of tube. After growing bacteria in medium containing the product, we measured the effect of it on biofilm production using crystal violet visually (Protocol). Biofilm production can also be quantified with TECAN and is proportional with absorbance. We measured fatty acid effect by adding it at the start of growth. Then we observed biofilm production after 24, 48, 72, and 96 hours.
These results are very encouraging. We can see a significant decrease of biofilm production after 48, 72 and 96 hours. We decided to replicate the mesure and quantify the experiments numerically.
Raw Datas:
All the measures were made by Tecan Infinite® 200 at a wavelenght of 570nm.
| Hours | 6 | 12 | 24 |
| Replicat1 | 0,33360001 | 0,44459999 | 0,78009999 |
| Replicat2 | 0,3337 | 0,48379999 | 0,91189998 |
| Replicat3 | 0,57870001 | 0,49349999 | 0,82230002 |
| Average | 0,41533334 | 0,47396666 | 0,8381 |
| Ecart-type | 0,14147969 | 0,02589061 | 0,06730557 |
| Hours | 6 | 12 | 24 |
| Replicat1 | 0,32260001 | 0,54210001 | 1,16499996 |
| Replicat2 | 0,456 | 0,50389999 | 0,82029998 |
| Replicat3 | 0,50999999 | 0,40290001 | 0,80269998 |
| Average | 0,42953333 | 0,48296667 | 0,92933331 |
| Ecart-type | 0,0964627 | 0,07192227 | 0,20428294 |
The second experiment consist in adding the product not directly in the medium but after a certain time of growth. We choose to add the fatty acid in a culture of 24 hours. We observed the effect at time 6 hours and 24 hours later.
| Hours | 6 | 24 |
| Replicat1 | 0,97390002 | 2,08780003 |
| Replicat2 | 0,97680002 | 1,68789995 |
| Replicat3 | 0,99870002 | 1,73870003 |
| Average | 0,98313336 | 1,83813334 |
| Ecart-type | 0,01355888 | 0,21770451 |
| Hours | 6 | 24 |
| Replicat1 | 0,96560001 | 1,44819999 |
| Replicat2 | 1,00259995 | 1,79219997 |
| Replicat3 | 0,9734 | 1,30620003 |
| Average | 0,98053332 | 1,51553333 |
| Ecart-type | 0,01950416 | 0,24989862 |
Contrary to a first visual quantification, results are inconclusive. Actually, there is not a significant difference beetween standard conditions and the add of fatty acid.
However, we noted a small reduction when the fatty acid is used after a period of growth. This difference let us think that maybe the fatty acid have a stronger impact on the current production.
Finally, visuals results bring to light some impact which deserve more research with different parameters.
References
- ↑ Rutherford, S. T. & Bassler, B. L. Bacterial Quorum Sensing: Its Role in Virulence and Possibilities for Its Control. Cold Spring Harb Perspect Med 2, a012427 (2012).
- ↑ Ionescu, M. et al. Promiscuous Diffusible Signal Factor Production and Responsiveness of the Xylella fastidiosa Rpf System. mBio 7, e01054–16 (2016).
- ↑ Amari, D. T., Marques, C. N. H. & Davies, D. G. The Putative Enoyl-Coenzyme A Hydratase DspI Is Required for Production of the Pseudomonas aeruginosa Biofilm Dispersion Autoinducer cis-2-Decenoic Acid. J. Bacteriol. 195, 4600–4610 (2013).