
Figure (1): Results of the analysis of PtNTT2 using Phobius.
The 30 first amino acids are clearly recognized as a signal peptide. Ten transmembrane domains are predicted.
| Line 541: | Line 541: | ||
The final OD<sub>600</sub> values varied widely, with <i>E. coli</i> BL21(DE3) pSB1C3-PlacUV5-pelB-SP-PtNTT2 reaching the highest OD<sub>600</sub> of 3.907 ± 0.018. The lowest OD<sub>600</sub> was reached by <i>E. coli</i> BL21(DE3) pSB1C3-PlacUV5-PtNTT2 with a value of 1.537 ± 0.045. All final optical densities at 600 nm are shown in table (7). | The final OD<sub>600</sub> values varied widely, with <i>E. coli</i> BL21(DE3) pSB1C3-PlacUV5-pelB-SP-PtNTT2 reaching the highest OD<sub>600</sub> of 3.907 ± 0.018. The lowest OD<sub>600</sub> was reached by <i>E. coli</i> BL21(DE3) pSB1C3-PlacUV5-PtNTT2 with a value of 1.537 ± 0.045. All final optical densities at 600 nm are shown in table (7). | ||
<br> | <br> | ||
| − | The cultivations were performed in parallel in MOPS media supplemented with 1 mM ATP as sole phosphate source. Again, three biological replicates of each strain were cultivated and three technical replicates measured for each time point. The growth curves are shown in figure ( | + | The cultivations were performed in parallel in MOPS media supplemented with 1 mM ATP as sole phosphate source. Again, three biological replicates of each strain were cultivated and three technical replicates measured for each time point. The growth curves are shown in figure (10). |
<div class="figure seventy"> | <div class="figure seventy"> | ||
<img class="figure image" src="https://static.igem.org/mediawiki/2017/1/14/T--Bielefeld-CeBiTec--microcultivation_ATP.jpeg"> | <img class="figure image" src="https://static.igem.org/mediawiki/2017/1/14/T--Bielefeld-CeBiTec--microcultivation_ATP.jpeg"> | ||
| − | <p class="figure subtitle"><b>Figure ( | + | <p class="figure subtitle"><b>Figure (10): Cultivation of all strains in MOPS media with 1 mM ATP acting as the sole phosphate source.</b><br> Three biological replicates were cultivated and three technical replicates measured for each time point.</p> |
</div> | </div> | ||
| Line 617: | Line 617: | ||
</table> | </table> | ||
| − | The maximum specific growth rate was graphically determined for all cultures as shown in figure ( | + | The maximum specific growth rate was graphically determined for all cultures as shown in figure (11). |
<div class="figure seventy"> | <div class="figure seventy"> | ||
<img class="figure image" src="https://static.igem.org/mediawiki/2017/3/3c/T--Bielefeld-CeBiTec--%C2%B5maxK2HPO4.jpeg"> | <img class="figure image" src="https://static.igem.org/mediawiki/2017/3/3c/T--Bielefeld-CeBiTec--%C2%B5maxK2HPO4.jpeg"> | ||
| − | <p class="figure subtitle"><b>Figure ( | + | <p class="figure subtitle"><b>Figure (11): Graphical determination of the maximum specific growth rates for all cultures cultivated in MOPS media with 1.32 mM K<sub>2</sub>HPO<sub>4</sub>.</b><br></p> |
</div> | </div> | ||
Based on the maximum specific growth rates, the minimum doubling time was calculated. The results are shown in table (8). Once again, the strains expressing the native transporter and the transporter with a TAT signal peptide showed the weakest growth. The best growth characteristics were achieved by the strains expressing the truncated versions <i>Pt</i>NTT2(66-575) and <i>Pt</i>NTT2(31-575), and <i>Pt</i>NTT2 with a pelB signal peptide. | Based on the maximum specific growth rates, the minimum doubling time was calculated. The results are shown in table (8). Once again, the strains expressing the native transporter and the transporter with a TAT signal peptide showed the weakest growth. The best growth characteristics were achieved by the strains expressing the truncated versions <i>Pt</i>NTT2(66-575) and <i>Pt</i>NTT2(31-575), and <i>Pt</i>NTT2 with a pelB signal peptide. | ||
| Line 691: | Line 691: | ||
</tbody> | </tbody> | ||
</table><br> | </table><br> | ||
| − | The graphical determination of the maximum specific growth rates of the cultures cultivated in ATP supplemented media is shown in figure ( | + | The graphical determination of the maximum specific growth rates of the cultures cultivated in ATP supplemented media is shown in figure (12). |
<div class="figure seventy"> | <div class="figure seventy"> | ||
<img class="figure image" src="https://static.igem.org/mediawiki/2017/b/bf/T--Bielefeld-CeBiTec--%C2%B5maxATP.jpeg"> | <img class="figure image" src="https://static.igem.org/mediawiki/2017/b/bf/T--Bielefeld-CeBiTec--%C2%B5maxATP.jpeg"> | ||
| − | <p class="figure subtitle"><b>Figure ( | + | <p class="figure subtitle"><b>Figure (12): Graphical determination of the maximum specific growth rates of all cultivations performed in MOPS media and 1 mM ATP. </b><br> </p> |
</div> | </div> | ||
The determined values for µmax and the minimal doubling times are shown in table (8). | The determined values for µmax and the minimal doubling times are shown in table (8). | ||
| Line 772: | Line 772: | ||
</div> | </div> | ||
<br> | <br> | ||
| − | For each measurement point <i>n</i>, the optical density at 600 nm of the cultivation of a transporter variant in ATP supplemented media (<i>OD<sub>AT</sub></i>) was divided by the optical density of the reference <i>E. coli</i> BL21(DE3) pSB1C3-PtNTT2 in ATP supplemented media (<i>OD<sub>AR</sub></i>). The same was done for the cultures in K<sub>2</sub>HPO<sub>4</sub> supplemented media(<i>OD<sub>KT</sub></i> and <i>OD<sub>KR</sub></i>). The value for ATP was then divided by the value for K<sub>2</sub>HPO<sub>4</sub>. The sum of the quotient for all measurement points <i>n</i> was then divided by <i>n</i> to obtain the final value for the relative beneficial effect of <i>Pt</i>NTT2 shown in figure ( | + | For each measurement point <i>n</i>, the optical density at 600 nm of the cultivation of a transporter variant in ATP supplemented media (<i>OD<sub>AT</sub></i>) was divided by the optical density of the reference <i>E. coli</i> BL21(DE3) pSB1C3-PtNTT2 in ATP supplemented media (<i>OD<sub>AR</sub></i>). The same was done for the cultures in K<sub>2</sub>HPO<sub>4</sub> supplemented media(<i>OD<sub>KT</sub></i> and <i>OD<sub>KR</sub></i>). The value for ATP was then divided by the value for K<sub>2</sub>HPO<sub>4</sub>. The sum of the quotient for all measurement points <i>n</i> was then divided by <i>n</i> to obtain the final value for the relative beneficial effect of <i>Pt</i>NTT2 shown in figure (13). The final error was calculated through error propagation of the standard error of each measured optical density. |
<div class="figure seventy"> | <div class="figure seventy"> | ||
<img class="figure image" src="https://static.igem.org/mediawiki/2017/4/40/T--Bielefeld-CeBiTec--RBE-1mM.jpeg"> | <img class="figure image" src="https://static.igem.org/mediawiki/2017/4/40/T--Bielefeld-CeBiTec--RBE-1mM.jpeg"> | ||
| − | <p class="figure subtitle"><b>Figure ( | + | <p class="figure subtitle"><b>Figure (13): Relative beneficial effect of the different <i>Pt</i>NTT2 variants.</b><br> As expected, the native transporter variant shows the highest positive effect since it most likely also exhibits the highest activity. Surprisingly, the two truncated versions show a higher effect than the versions with a pelB and TAT signal peptide.</p> |
</div> | </div> | ||
Of all transporter variants, the native <i>Pt</i>NTT2 showed the highest beneficial effect with a value of 1.360 ± 0.161. The second highest value was reached by <i>Pt</i>NTT2(31-575) with 1.294 ± 0.107. <i>Pt</i>NTT2(66-575) reached a value of 1.166 ± 0.105, pelB-SP-PtNTT2 one of 1.073 ± 0.114 and TAT-SP-PtNTT2 one of 0.732 ± 0.116. | Of all transporter variants, the native <i>Pt</i>NTT2 showed the highest beneficial effect with a value of 1.360 ± 0.161. The second highest value was reached by <i>Pt</i>NTT2(31-575) with 1.294 ± 0.107. <i>Pt</i>NTT2(66-575) reached a value of 1.166 ± 0.105, pelB-SP-PtNTT2 one of 1.073 ± 0.114 and TAT-SP-PtNTT2 one of 0.732 ± 0.116. | ||
| Line 793: | Line 793: | ||
<article> | <article> | ||
| − | To investigate the subcellular localization of the different <i>Pt</i>NTT2 variants, GFP fusion proteins were constructed. Each <i>Pt</i>NTT2 variant was tagged with a cMyc epitope tag as a linker and GFP (BBa_E0040). The cells were cultivated and prepared for microscopy as described in the methods section. Microscopy was performed using the Zeiss LSM 700. The pictures shown in figure ( | + | To investigate the subcellular localization of the different <i>Pt</i>NTT2 variants, GFP fusion proteins were constructed. Each <i>Pt</i>NTT2 variant was tagged with a cMyc epitope tag as a linker and GFP (BBa_E0040). The cells were cultivated and prepared for microscopy as described in the methods section. Microscopy was performed using the Zeiss LSM 700. The pictures shown in figure (14) were taken at 100x magnification and clearly show the subcellular localization of the different <i>Pt</i>NTT2 variants. |
<div class="figure large"> | <div class="figure large"> | ||
<img class="figure image" src="https://static.igem.org/mediawiki/2017/a/a1/T--Bielefeld-CeBiTec--microscopy.jpeg"> | <img class="figure image" src="https://static.igem.org/mediawiki/2017/a/a1/T--Bielefeld-CeBiTec--microscopy.jpeg"> | ||
| − | <p class="figure subtitle"><b>Figure ( | + | <p class="figure subtitle"><b>Figure (14): Confocal laser scanning microscopy of the different <i>Pt</i>NTT2 variants fused to GFP (BBa_E0040). </b><br> The pictures were taken with 100x magnification and show from A to E: <i>E. coli</i> BL21(DE3), <i>E. coli</i> BL21(DE3) pSB1C3-PtNTT2, <i>E. coli</i> BL21(DE3) pSB1C3-PlacUV5-PtNTT2, <i>E. coli</i> BL21(DE3) pSB1C3-PlacUV5-PtNTT2(66-575), <i>E. coli</i> BL21(DE3) pSB1C3-PlacUV5-PtNTT2(31-575), <i>E. coli</i> BL21(DE3) pSB1C3-PlacUV5-pelB-SP-PtNTT2 and <i>E. coli</i> BL21(DE3) pSB1C3-PlacUV5-TAT-SP-PtNTT2.</p> |
</div> | </div> | ||
All <i>Pt</i>NTT2 variants can be localized within the membrane, since the fluorescence signals of the GFP tag are concentrated there. Judging from the pictures, the variant with the pelB signal peptide is weakly integrated into the inner membrane, which would explain the good growth characteristics and the weak functionality of this variant. The TAT-signal peptide variant, which showed bad growth characteristics and no function, seems to be located within the cell envelope nonetheless. Given that peptides containing the TAT signal peptide are usually folded within the cytoplasm and then secreted via the twin-arginine translocation pathway, this could be a possible explanation for the non-existent function of the TAT variant. All other variants that also showed a detectable function are successfully integrated into the membrane. The variants without a signal peptide are also located within the membrane, which is not surprising, given that the integration of membrane proteins does not necessarily require a signal peptide (Facey and Kuhn, 2004) . <i>Pt</i>NTT2(66-575) seems to be better integrated into the membrane than any other variant. The native <i>Pt</i>NTT2, and <i>Pt</i>NTT2(31-575) are integrated in a similar scale, but a little bit weaker compared to <i>Pt</i>NTT2(66-575). These results are consistent with the results of the verification of the function of <i>Pt</i>NTT2, since all variants that showed a relative beneficial effect and also the highest AMP concentrations in the supernatant do integrate the transporter into the membrane. For the TAT signal peptide variant, it is most likely that the transporter is integrated incorrectly, leading to a correct subcellular localization while lacking the correct function. | All <i>Pt</i>NTT2 variants can be localized within the membrane, since the fluorescence signals of the GFP tag are concentrated there. Judging from the pictures, the variant with the pelB signal peptide is weakly integrated into the inner membrane, which would explain the good growth characteristics and the weak functionality of this variant. The TAT-signal peptide variant, which showed bad growth characteristics and no function, seems to be located within the cell envelope nonetheless. Given that peptides containing the TAT signal peptide are usually folded within the cytoplasm and then secreted via the twin-arginine translocation pathway, this could be a possible explanation for the non-existent function of the TAT variant. All other variants that also showed a detectable function are successfully integrated into the membrane. The variants without a signal peptide are also located within the membrane, which is not surprising, given that the integration of membrane proteins does not necessarily require a signal peptide (Facey and Kuhn, 2004) . <i>Pt</i>NTT2(66-575) seems to be better integrated into the membrane than any other variant. The native <i>Pt</i>NTT2, and <i>Pt</i>NTT2(31-575) are integrated in a similar scale, but a little bit weaker compared to <i>Pt</i>NTT2(66-575). These results are consistent with the results of the verification of the function of <i>Pt</i>NTT2, since all variants that showed a relative beneficial effect and also the highest AMP concentrations in the supernatant do integrate the transporter into the membrane. For the TAT signal peptide variant, it is most likely that the transporter is integrated incorrectly, leading to a correct subcellular localization while lacking the correct function. | ||
| Line 816: | Line 816: | ||
<article> | <article> | ||
For further analysis of <i>Pt</i>NTT2 the isolation of the transporter from the cell was attempted. This task proved to be challenging, given that <i>Pt</i>NTT2 comprises ten transmembrane domains and is therefore highly hydrophobic. For that reason, several different methods and procedures were tested to achieve the best results. | For further analysis of <i>Pt</i>NTT2 the isolation of the transporter from the cell was attempted. This task proved to be challenging, given that <i>Pt</i>NTT2 comprises ten transmembrane domains and is therefore highly hydrophobic. For that reason, several different methods and procedures were tested to achieve the best results. | ||
| − | The first two attempts were based on the isolation of the periplasmic protein fraction through cold osmotic shock and fast cell lysis followed by analysis via SDS PAGE. Unsurprisingly, both methods did not lead to the desired results, since not bands for <i>Pt</i>NTT2 could be observed. Figure ( | + | The first two attempts were based on the isolation of the periplasmic protein fraction through cold osmotic shock and fast cell lysis followed by analysis via SDS PAGE. Unsurprisingly, both methods did not lead to the desired results, since not bands for <i>Pt</i>NTT2 could be observed. Figure (15) shows the SDS PAGE of the samples prepared using the fast cell lysis for SDS PAGE. |
<div class="figure sixty"> | <div class="figure sixty"> | ||
<img class="figure image" src="https://static.igem.org/mediawiki/2017/3/34/T--Bielefeld-CeBiTec--SDSPAGEkochen.jpeg"> | <img class="figure image" src="https://static.igem.org/mediawiki/2017/3/34/T--Bielefeld-CeBiTec--SDSPAGEkochen.jpeg"> | ||
| − | <p class="figure subtitle"><b>Figure ( | + | <p class="figure subtitle"><b>Figure (15): SDS-PAGE of the GFP-fusion constructs of <i>Pt</i>NTT2</b><br> The cells were prepared using the fast cell lysis for SDS PAGE protocol. <i>E. coli</i> BL21(DE3) and <i>E. coli</i> BL21(DE3) pSB1C3-PtNTT2 were used as negative controls. Unsurprisingly, no thick band can be observed around 90.3 kDa, which would be the size of <i>Pt</i>NTT2-cMyc-GFP. No bands can be observed for the other <i>Pt</i>NTT2 variants. </p> |
</div> | </div> | ||
| − | A second gel was used for a western blot using an anti-GFP antibody. The result is shown in figure ( | + | A second gel was used for a western blot using an anti-GFP antibody. The result is shown in figure (16). |
<div class="figure sixty"> | <div class="figure sixty"> | ||
<img class="figure image" src="https://2017.igem.org/wiki/images/f/ff/T--Bielefeld-CeBiTec--WesternBlotkochen.jpeg"> | <img class="figure image" src="https://2017.igem.org/wiki/images/f/ff/T--Bielefeld-CeBiTec--WesternBlotkochen.jpeg"> | ||
| − | <p class="figure subtitle"><b>Figure ( | + | <p class="figure subtitle"><b>Figure (16): Western Blot of the samples prepared using the fast cell lysis for SDS PAGE protocol. </b><br> An anti-GFP antibody was used for the detection of <i>Pt</i>NTT2-cMyc-GFP variants. E . coli BL21(DE3) and <i>E. coli</i> BL21(DE3) pSB1C3-PtNTT2 were used as negative controls. Much unspecific binding of the anti-GFP antibody could be observed, which is not surprising given that the entire proteome of the cells was analyzed. Thick band can be observed for <i>Pt</i>NTT2-cMyc-GFP, <i>Pt</i>NTT2(66-575)-cMyc-GFP, <i>Pt</i>NTT2(31-575)-cMyc-GFP and <i>Pt</i>NTT2(pelB)-cMyc-GFP around 35 kDa. This indicates that only the cMyc-GFP linker was detected and that the linker might be cleaved of from <i>Pt</i>NTT2 due to the high difference in hydrophobicity. </p> |
</div> | </div> | ||
| − | In a second approach, the cells were lysed using a nitrogen cooled Precellys Homogenizer. After three cycles, cells were centrifuged at 4 °C and the membrane fraction was isolated as described in the methods section . After boiling the membrane fraction, the samples were loaded onto two SDS PAGEs, one of which was consequently used for a western blot, if the fusion proteins were used. For the western blot, two different antibodies were tested, an anti-GFP antibody and an anti-cMyc antibody. Figure ( | + | In a second approach, the cells were lysed using a nitrogen cooled Precellys Homogenizer. After three cycles, cells were centrifuged at 4 °C and the membrane fraction was isolated as described in the methods section . After boiling the membrane fraction, the samples were loaded onto two SDS PAGEs, one of which was consequently used for a western blot, if the fusion proteins were used. For the western blot, two different antibodies were tested, an anti-GFP antibody and an anti-cMyc antibody. Figure (17) shows the SDS-PAGE after isolation of the membrane fraction. Again, no clear difference between the negative controls and the samples could be observed around 90 kDa. |
<div class="figure sixty"> | <div class="figure sixty"> | ||
<img class="figure image" src="https://static.igem.org/mediawiki/2017/b/bf/T--Bielefeld-CeBiTec--SDSPAGEribo.jpeg"> | <img class="figure image" src="https://static.igem.org/mediawiki/2017/b/bf/T--Bielefeld-CeBiTec--SDSPAGEribo.jpeg"> | ||
| − | <p class="figure subtitle"><b>Figure ( | + | <p class="figure subtitle"><b>Figure (17): SDS PAGE performed with the isolated membrane fractions.</b><br> The cMyc-GFP fusion proteins were used, which should be visible around ~90 kDa, differing slightly based on the <i>Pt</i>NTT2 variant used. No bands could be observed around 90 kDa, which was subsequently proofed by performing a western blot. </p> |
</div> | </div> | ||
| − | The western blot (figure | + | The western blot (figure 18) performed with the same samples verified these results, since only bands around ~28 kDa could be observed for all samples except for <i>Pt</i>NTT2(TAT)-cMyc-GFP and not for the negative controls. These bands can again be associated with the cMyc-GFP tag, providing further evidence that the tag can be cleaved of from the transporter. Again, no band could be observed for <i>Pt</i>NTT2-cMyc-GFP, indicating that <i>Pt</i>NTT2 with a TAT signal peptide is weakly integrated into the membrane, which would explain the lacking function of this variant. The weak growth characteristics of the strain producing <i>Pt</i>NTT2 with a TAT signal peptide would therefore most likely be caused by accumulation of the protein within the cell. |
<div class="figure sixty"> | <div class="figure sixty"> | ||
<img class="figure image" src="https://static.igem.org/mediawiki/2017/e/ec/T--Bielefeld-CeBiTec--WesternBlotRibo.jpeg"> | <img class="figure image" src="https://static.igem.org/mediawiki/2017/e/ec/T--Bielefeld-CeBiTec--WesternBlotRibo.jpeg"> | ||
| − | <p class="figure subtitle"><b>Figure ( | + | <p class="figure subtitle"><b>Figure (18): Western Blot of the isolated membrane fractions of the strains expressing the cMyc-GFP fusion proteins.</b><br> Thick bands can be observed around 28 kDa for all samples except for <i>Pt</i>NTT2-cMyc-GFP with a TAT signal peptide. The negative controls do not show the same band, but some unspecific binding of the anti-GFP antibody could be observed. Compared to the previous western blot, unspecific binding was significantly reduced. These results indicate, that the linker is most likely separated from the transporter either during the isolation process or already within the cell. This would be no surprise, given that the transporter is highly hydrophobic while the linker is hydrophilic. </p> |
</div> | </div> | ||
| Line 850: | Line 850: | ||
<div class="figure sixty"> | <div class="figure sixty"> | ||
<img class="figure image" src="https://static.igem.org/mediawiki/2017/5/51/T--Bielefeld-CeBiTec--WesternBlotcmycribo.jpeg"> | <img class="figure image" src="https://static.igem.org/mediawiki/2017/5/51/T--Bielefeld-CeBiTec--WesternBlotcmycribo.jpeg"> | ||
| − | <p class="figure subtitle"><b>Figure ( | + | <p class="figure subtitle"><b>Figure (19): Western Blot of the isolated membrane fraction using an anti-cMyc antibody. </b><br> Again, fragments can be observed around 35 kDa for all samples except for <i>Pt</i>NTT2-cMyc-GFP with a TAT signal peptide. No bands can be observed for the full construct, but a very weak band can be seen between 55 and 70 kDa for the fusion protein of the native transporter. </p> |
</div> | </div> | ||
| Line 859: | Line 859: | ||
<div class="figure sixty"> | <div class="figure sixty"> | ||
<img class="figure image" src="https://static.igem.org/mediawiki/2017/2/2b/T--Bielefeld-CeBiTec--SDSPAGEnoboiling.jpeg"> | <img class="figure image" src="https://static.igem.org/mediawiki/2017/2/2b/T--Bielefeld-CeBiTec--SDSPAGEnoboiling.jpeg"> | ||
| − | <p class="figure subtitle"><b>Figure ( | + | <p class="figure subtitle"><b>Figure (20): SDS PAGE of the isolated membrane fraction without previous boiling</b><br> No thick bands can be observed around 70 kDa. Slightly above 100 kDa, bands can be observed for all <i>Pt</i>NTT2 variants but not for the negative controls. But given that the samples ran quite different on the gel compared to the boiled samples, no definite conclusion can be drawn from this gel. </p> |
</div> | </div> | ||






Figure (1): Results of the analysis of PtNTT2 using Phobius.
The 30 first amino acids are clearly recognized as a signal peptide. Ten transmembrane domains are predicted.

Figure (2): Schematic overview of the design of the different transporter variants.
The lacUV5 promotor was used together with a strong RBS (BBa_B0034) for all parts. All variants except for pSB1C3-PtNTT2 were also tagged with GFP (BBa_E0040). cMyc was used as a linker (based on BBa_K2082221).
Table (1): Designed and cloned plasmids for the analysis and characterization of PtNTT2.
| Plasmid Name | BioBrick Number | Characteristics | |
|---|---|---|---|
| pSB1C3-PtNTT2 | BBa_K2201004 | Only the cds | |
| pSB1C3-PlacUV5-PtNTT2(66-575) | BBa_ K2201001 | cds with lacUV5 promotor and a strong RBS (BBa_B0034) | |
| pSB1C3-PlacUV5-PtNTT2(31-575) | BBa_K2201005 | cds with lacUV5 promotor and a strong RBS (BBa_B0034), truncated version lacking the first 30 amino acids | |
| pSB1C3-PlacUV5-pelB-SP-PtNTT2 | BBa_K2201006 | cds with lacUV5 promotor and a strong RBS (BBa_B0034), native signal peptide replaced with the pelB signal peptide | |
| pSB1C3-PlacUV5-TAT-SP-PtNTT2 | BBa_K2201007 | cds with lacUV5 promotor and a strong RBS (BBa_B0034), native signal peptide replaced with a TAT signal peptide | |
| pSB1C3-PlacUV5-PtNTT2-GFP | BBa_K2201002 | Fusion protein of BBa_ K2201000 with GFP (BBa_E0040), Myc epitope tag as linker (BBa_K2201181) | |
| pSB1C3-PlacUV5-PtNTT2(66-575)-GFP | BBa_K2201003 | Fusion protein of BBa_ K2201001 with GFP (BBa_E0040), Myc epitope tag as linker (BBa_K2201181) | |
| pSB1C3-PlacUV5-PtNTT2(31-575)-GFP | BBa_K2201011 | Fusion protein of BBa_K2201005 with GFP (BBa_E0040), Myc epitope tag as linker (BBa_K2201181) | |
| pSB1C3-PlacUV5-pelB-SP-PtNTT2-GFP | BBa_K2201012 | Fusion protein of BBa_K2201006 with GFP (BBa_E0040), Myc epitope tag as linker (BBa_K2201181) | |
| pSB1C3-PlacUV5-TAT-SP-PtNTT2-GFP | BBa_K2201013 | Fusion protein of BBa_K2201007 with GFP (BBa_E0040), Myc epitope tag as linker (BBa_K2201181) |

Figure (3): Shake flask cultivation of all PtNTT2 variants.
E. coli BL21(DE3) and E. coli BL21(DE3) pSB1C3-PtNTT2, not expressing PtNTT2, were used as negative controls. Two biological replicates of each strain were cultivated and three technical replicates taken for each measurement. A clear difference in the growth rates can be observed, with E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2 and E. coli BL21(DE3) pSB1C3-PlacUV5-TAT-SP-PtNTT2 showing the weakest growth. Both strains also show the longest lag phase, which is nearly twice as long as the lag phase of E. coli BL21(DE3). E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(66-575) and E. coli BL21(DE3) pSB1C3-PlacUV5-pelB-SP-PtNTT2 show the best growth of all PtNTT2 variants, reaching the highest OD600.
Table (2): Final OD600 of all cultures.
The highest OD600 was reached by the wildtype E. coli BL21(DE3), the lowest by E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2.
| Strain | Final OD600 [-] | |
|---|---|---|
| E. coli BL21(DE3) | 5.178 ± 0.046 | |
| E. coli BL21(DE3) pSB1C3-PtNTT2 | 4.638 ± 0.029 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2 | 2.499 ± 0.134 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(66-575) | 4.397 ± 0.062 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(31-575) | 3.802 ± 0.135 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-pelB-SP-PtNTT2 | 4.171 ± 0.051 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-TAT-SP-PtNTT2 | 2.735 ± 0.150 |

Figure (4): Graphical determination of µmax.
The highest specific growth rate was determined for each culture by plotting the natural logarithm of OD600 against the cultivation time. The slope of the linear regression through the exponential phase gives µmax.

(1)
Table (3): Maximum specific growth rates and minimum doubling times for all cultures.
| Strain | µmax [h-1] | td [h] | |
|---|---|---|---|
| E. coli BL21(DE3) | 1.201 ± 0.070 | 0.577 ± 0.058 | |
| E. coli BL21(DE3) pSB1C3-PtNTT2 | 1.212 ± 0.029 | 0.572 ± 0.024 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2 | 0.978 ± 0.033 | 0.709 ± 0.034 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(66-575) | 1.194 ± 0.026 | 0.581 ± 0.022 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(31-575) | 1.143 ± 0.045 | 0.606 ± 0.039 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-pelB-SP-PtNTT2 | 1.189 ± 0.028 | 0.583 ± 0.024 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-TAT-SP-PtNTT2 | 0.946 ± 0.030 | 0.733 ± 0.032 |

Figure (5): Microcultivation of all PtNTT2 variants
E. coli BL21(DE3) and E. coli BL21(DE3) pSB1C3-PtNTT2 (BBa_K2201004) were again used as negative controls. The same growth pattern as in the shake flask cultivation can be observed, with E. coli BL21(DE3) pSB1C3-PlacUV5-pelB-SP-PtNTT2, E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(66-575) reaching the highest ODs, followed by E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(31-575), E. coli BL21(DE3) pSB1C3-PlacUV5-TAT-SP-PtNTT2 and E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2.
Table (4): Final OD600 of all cultures.
The highest OD600 was reached by the wildtype E. coli BL21(DE3) with 5,487 ± 0.038, the lowest by E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2 with 1.623 ± 0.481.
| Strain | Final OD600 [-] | |
|---|---|---|
| E. coli BL21(DE3) | 5.487 ± 0.038 | |
| E. coli BL21(DE3) pSB1C3-PtNTT2 | 4.337 ± 0.010 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2 | 1.623 ± 0.481 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(66-575) | 4.035 ± 0.051 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(31-575) | 3.865 ± 0.008 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-pelB-SP-PtNTT2 | 4.110 ± 0.005 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-TAT-SP-PtNTT2 | 2.280 ± 0.337 |

Figure (6): Graphical determination of the maximum specific growth rate µmax for the microcultivations.
Table (5): Maximum specific growth rate and minimum doubling time for all cultures cultivated in 12 well plates.
| Strain | µmax [h-1] | td [h] | |
|---|---|---|---|
| E. coli BL21(DE3) | 1.059 ± 0.143 | 0.655 ± 0.135 | |
| E. coli BL21(DE3) pSB1C3-PtNTT2 | 1.016 ± 0.133 | 0.682 ± 0.131 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2 | 0.829 ± 0.071 | 0.836 ± 0.086 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(66-575) | 1.023 ± 0.105 | 0.678 ± 0.103 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(31-575) | 1.021 ± 0.096 | 0.679 ± 0.094 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-pelB-SP-PtNTT2 | 1.047 ± 0.097 | 0.662 ± 0.093 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-TAT-SP-PtNTT2 | 0.924 ± 0.113 | 0.750 ± 0.122 |

Figure (7): Microcultivation in a 96 well plate performed by iGEM team UNIFI from Florence, Italy.
E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2 and E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(66-575) were cultivated in a total volume of 3 mL at 37 °C and 130 rpm. The growth difference between the two strains observed in previous cultivations could also be observed in this experiment carried out by the team from Florence. E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2 reached a final OD600 of 0.329 ± 0.037 while E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(66-575) reached a final OD600 of 0.664 ± 0.033.

Figure (8): Graphical determination of the maximum specific growth rates for the cultivations carried out in 96 well plates by the iGEM team UNIFI.
Table (6): Maximum specific growth rate and minimum doubling time for all cultures cultivated in 12 well plates.
| Strain | µmax [h-1] | td [h] | |
|---|---|---|---|
| E. coli BL21(DE3) | 0.042 ± 0.004 | 16.504 ± 0.095 | |
| E. coli BL21(DE3) pSB1C3-PtNTT2 | 0.110 ± 0.002 | 6.301 ± 0.018 |

Figure (9): Cultivation of all transporter variants in MOPS media with K2HPO4 acting as the sole phosphate source.
The cultivation was carried out in 12 well plates and three biological replicates were cultivated of each strain. For measurement of the optical density at 600 nm, three technical replicates were taken.

Figure (10): Cultivation of all strains in MOPS media with 1 mM ATP acting as the sole phosphate source.
Three biological replicates were cultivated and three technical replicates measured for each time point.
Table (7): Final OD600 values for all cultivations carried out in MOPS media with 1,32 mM K2HPO4.
| Strain | Final OD600, K2HPO4 [-] | Final OD600, ATP [-] | |
|---|---|---|---|
| E. coli BL21(DE3) | 2.923 ± 0.028 | 4.967 ± 0.143 | |
| E. coli BL21(DE3) pSB1C3-PtNTT2 | 3.507 ± 0.048 | 3.673 ± 0.091 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2 | 1.537 ± 0.045 | 3.033 ± 0.028 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(66-575) | 3.560 ± 0.011 | 3.347 ± 0.032 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(31-575) | 3.797 ± 0.065 | 3.580 ± 0.006 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-pelB-SP-PtNTT2 | 3.907 ± 0.018 | 3.710 ± 0.177 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-TAT-SP-PtNTT2 | 3.307 ± 0.029 | 2.177 ± 0.007 |

Figure (11): Graphical determination of the maximum specific growth rates for all cultures cultivated in MOPS media with 1.32 mM K2HPO4.
Table (8): Maximum specific growth rates and minimal doubling times of the cultivations in MOPS media with 1.32 mM K2HPO4.
| Strain | µmax [h-1] | td [h] | |
|---|---|---|---|
| E. coli BL21(DE3) | 0.444 ± 0.053 | 1.561 ± 0.199 | |
| E. coli BL21(DE3) pSB1C3-PtNTT2 | 0.499 ± 0.050 | 1.389 ± 0.100 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2 | 0.385 ± 0.044 | 1.800 ± 0.114 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(66-575) | 0,568 ± 0.057 | 1.220 ± 0.100 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(31-575) | 0.532 ± 0.022 | 1.303 ± 0.041 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-pelB-SP-PtNTT2 | 0.549 ± 0.017 | 1.263 ± 0.031 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-TAT-SP-PtNTT2 | 0.463 ± 0.028 | 1.497 ± 0.060 |

Figure (12): Graphical determination of the maximum specific growth rates of all cultivations performed in MOPS media and 1 mM ATP.
Table (8): Maximum specific growth rates and minimal doubling times of the cultivations in MOPS media with 1 mM ATP.
| Strain | µmax [h-1] | td [h] | |
|---|---|---|---|
| E. coli BL21(DE3) | 0.673 ± 0.012 | 1.030 ± 0.018 | |
| E. coli BL21(DE3) pSB1C3-PtNTT2 | 0.600 ± 0.021 | 1.155 ± 0.035 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2 | 0.463 ± 0.035 | 1.497 ± 0.076 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(66-575) | 0.644 ± 0.069 | 1.076 ± 0.107 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(31-575) | 0.428 ± 0.091 | 1.620 ± 0.213 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-pelB-SP-PtNTT2 | 0.518 ± 0.043 | 1.338 ± 0.083 | |
| E. coli BL21(DE3) pSB1C3-PlacUV5-TAT-SP-PtNTT2 | 0.334 ± 0.047 | 2.075 ± 0.141 |

(2)

Figure (13): Relative beneficial effect of the different PtNTT2 variants.
As expected, the native transporter variant shows the highest positive effect since it most likely also exhibits the highest activity. Surprisingly, the two truncated versions show a higher effect than the versions with a pelB and TAT signal peptide.

Figure (14): Confocal laser scanning microscopy of the different PtNTT2 variants fused to GFP (BBa_E0040).
The pictures were taken with 100x magnification and show from A to E: E. coli BL21(DE3), E. coli BL21(DE3) pSB1C3-PtNTT2, E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2, E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(66-575), E. coli BL21(DE3) pSB1C3-PlacUV5-PtNTT2(31-575), E. coli BL21(DE3) pSB1C3-PlacUV5-pelB-SP-PtNTT2 and E. coli BL21(DE3) pSB1C3-PlacUV5-TAT-SP-PtNTT2.

Figure (15): SDS-PAGE of the GFP-fusion constructs of PtNTT2
The cells were prepared using the fast cell lysis for SDS PAGE protocol. E. coli BL21(DE3) and E. coli BL21(DE3) pSB1C3-PtNTT2 were used as negative controls. Unsurprisingly, no thick band can be observed around 90.3 kDa, which would be the size of PtNTT2-cMyc-GFP. No bands can be observed for the other PtNTT2 variants.
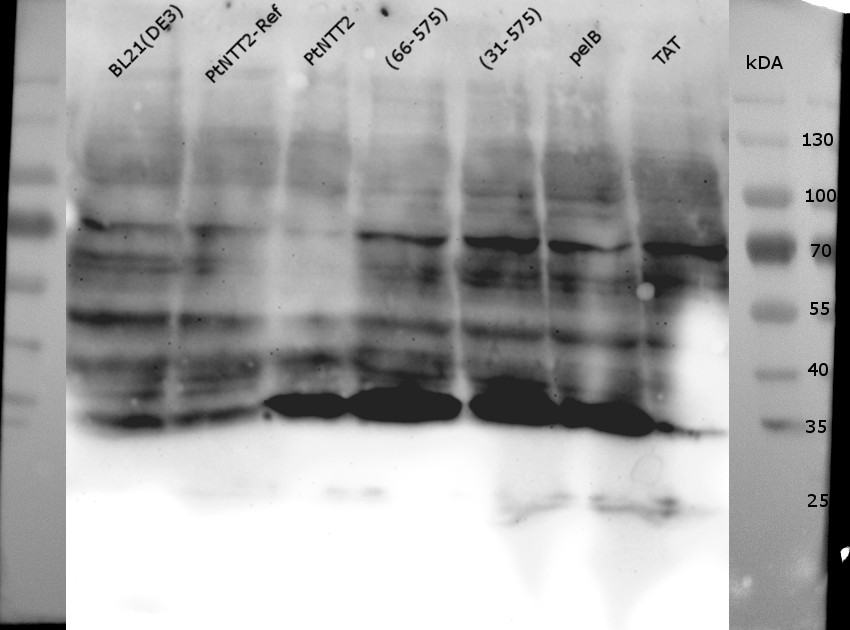
Figure (16): Western Blot of the samples prepared using the fast cell lysis for SDS PAGE protocol.
An anti-GFP antibody was used for the detection of PtNTT2-cMyc-GFP variants. E . coli BL21(DE3) and E. coli BL21(DE3) pSB1C3-PtNTT2 were used as negative controls. Much unspecific binding of the anti-GFP antibody could be observed, which is not surprising given that the entire proteome of the cells was analyzed. Thick band can be observed for PtNTT2-cMyc-GFP, PtNTT2(66-575)-cMyc-GFP, PtNTT2(31-575)-cMyc-GFP and PtNTT2(pelB)-cMyc-GFP around 35 kDa. This indicates that only the cMyc-GFP linker was detected and that the linker might be cleaved of from PtNTT2 due to the high difference in hydrophobicity.

Figure (17): SDS PAGE performed with the isolated membrane fractions.
The cMyc-GFP fusion proteins were used, which should be visible around ~90 kDa, differing slightly based on the PtNTT2 variant used. No bands could be observed around 90 kDa, which was subsequently proofed by performing a western blot.

Figure (18): Western Blot of the isolated membrane fractions of the strains expressing the cMyc-GFP fusion proteins.
Thick bands can be observed around 28 kDa for all samples except for PtNTT2-cMyc-GFP with a TAT signal peptide. The negative controls do not show the same band, but some unspecific binding of the anti-GFP antibody could be observed. Compared to the previous western blot, unspecific binding was significantly reduced. These results indicate, that the linker is most likely separated from the transporter either during the isolation process or already within the cell. This would be no surprise, given that the transporter is highly hydrophobic while the linker is hydrophilic.

Figure (19): Western Blot of the isolated membrane fraction using an anti-cMyc antibody.
Again, fragments can be observed around 35 kDa for all samples except for PtNTT2-cMyc-GFP with a TAT signal peptide. No bands can be observed for the full construct, but a very weak band can be seen between 55 and 70 kDa for the fusion protein of the native transporter.

Figure (20): SDS PAGE of the isolated membrane fraction without previous boiling
No thick bands can be observed around 70 kDa. Slightly above 100 kDa, bands can be observed for all PtNTT2 variants but not for the negative controls. But given that the samples ran quite different on the gel compared to the boiled samples, no definite conclusion can be drawn from this gel.
