
MODELING
Predict and optimize yield
Background
The purpose of modeling is to carefully examine the pathways of each intended biosynthetic product, look for ways to optimize production, and understand limiting factors. To accomplish these goals, we used available metabolic pathways for our target organism, and evaluated several different methods to model production of acetaminophen and B12 in cyanobacteria. Each of these modeling methods has different assumptions which allow these data to be averaged; providing reasonable quantitative estimates of our biosynthetic products.
ACETAMINOPHEN
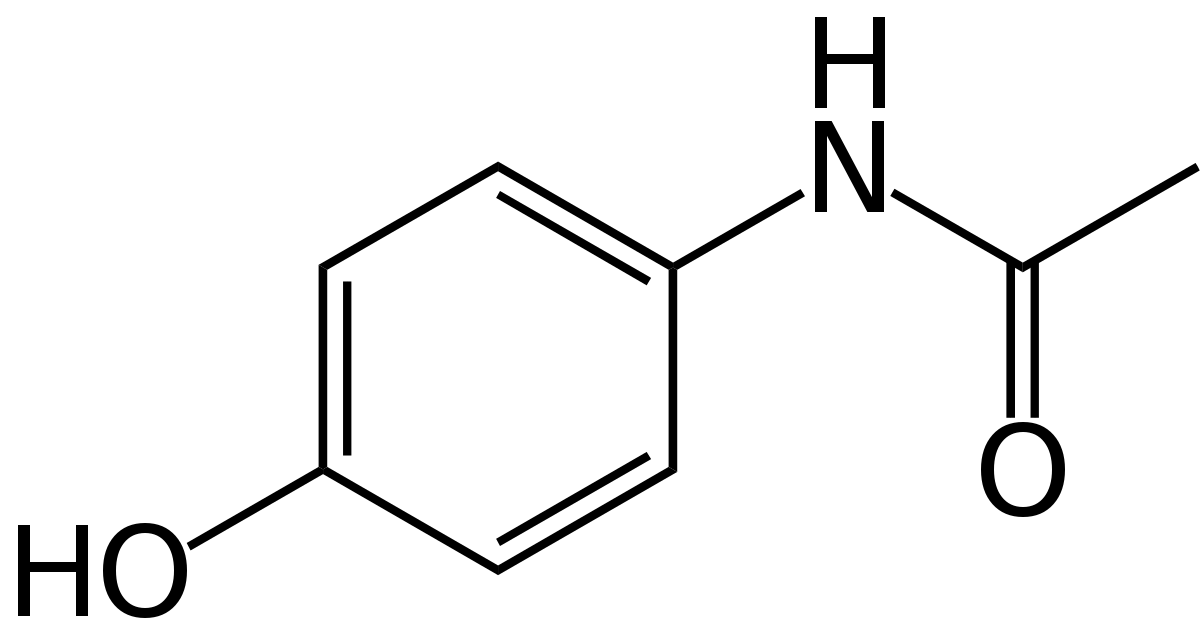
Overview
To predict acetaminophen biosynthesis, we analyzed the abundance of the acetaminophen's precursors anthranilate and PABA. Anthranilate comes from chorismate, which is primarily used by the cell to produce the aromatic amino acids phenylalanine, tyrosine and tryptophan, and in some organisms, salicylic acid, folate, vitamins, and alkaloids[1]. We used publicly available tryptophan and folate data, estimations from genomic sequences, and ribosomal protein sequences to estimate the available precursor material for our products. Combining this information with enzyme kinetics, we were able to model how much product was being produced per gram of biomass of our engineered Arthrospira platensis. Our results indicate that a reasonable sized culture could produce 200 doses of acetaminophen every 10 days.

Method using Published Tryptophan Data
Because chorismate is used as a precursor for the aromatic amino acids, we used the amino acid concentration as a proxy for the chorismate concentration. We used the amino acid and folate concentrations for A. platensis to calculate the moles per cell of the amino acid precursors anthranilate and PABA. These molecules are the direct substrate for our enzyme 4ABH, which converts anthranilate and PABA to the intermediate 4-aminophenol before nhoA converts that to acetaminophen. We show several different calculations below using different sources of data.
Assumptions
- Synechococcus and Arthrospira platensis have similar amino acid ratios. Since there was no available amino acid data for Synechococcus, we assumed it has a similar ratio to the more well described Arthrospira platensis.
- The amount of anthranilate and PABA precursors are equal to that of their products tryptophan and folate.
- Based on the Km ratios of 4ABH and its competitor TrpD, 33% of the available precursors will go down the acetaminophen pathway.
- Folate is present in such small amounts, at 0.21nM of PABA, that its effect on acetaminophen production is neglegible[7].
$$\frac{0.442\ µmol\ Trp}{1g\ biomass}\approx \frac{0.442\ µmol\ anth}{1g\ biomass}\times\frac{1\ mol\ acet}{3\ moles\ anth}\times\frac{151g\ acet.}{1 mol acet.}=\frac{2.3mg\ acet.}{1g\ biomass}$$
Sequence Analysis Method
To validate our organism's quantity of tryptophan precursor, we used a custom Python program[9] to convert DNA sequences to amino acids and calculate molar and mass percentages of tryptophan. We ran both the genome and all 55 listed ribosomal protein sequences through our program, which resulted in 0.9% and 0.6% tryptophan by moles. Knowing that about 60% of Arthrospira platensis is protein by mass, we can predict acetaminophen production.
$$\frac{0.054\ g\ Trp}{1\ biomass}\rightarrow\frac{0.27\ mmol\ Trp}{1\ g\ biomass}\times\frac{1\ mol\ acet}{3\ mol\ Trp}\times\frac{151.163g\ acet}{1\ mol\ acet.}=\frac{13.7mg\ acet.}{1g\ biomass}$$
$$\frac{0.036 g\ Trp}{1 g\ biomass}\rightarrow \frac{0.0.175\ mmol\ Trp}{1\ g\ biomass} * \frac{1\ mol\ acet}{3\ mol\ chor} *\frac{151.163\ g}{1 mol\ acet} = \frac{8.8\ mg\ acet}{1 g\ biomass}$$
Assumptions
- Codon composition from the genome and ribosomal proteins alone apprimates the amino acid composition of the cyanobacteria.
- The amount of anthranilate and PABA precursors are equal to that of their products tryptophan and folate.
- Of the available precursors, 33% will go down our pathway. This is based off the idea that the enzymes may have similar affinities for the precursor.
- Folate is present in such small amounts, at 0.21nM of PABA, that its effect on acetaminophen production is neglegible[7].
Enzyme Competition
Besides precursor concentrations, the main limiting factor would be how effective our enzyme 4ABH was at converting precursors from the tryptophan and folate pathways into 4-aminophenol. We can either assume all three competing enzymes will have similar precursor affinity and produce each product in equal quantities or use ratios of each competing enzyme's Km for the limiting intermediates. To make up for unknown enzyme rates and quantities, we made a chorismate metabolic simulation which ultimately used unreliable Km ratios. For that reason, all of the following calulations will be computed using a 33% precursor to product conversion rate.
Enzyme Competition Details
Since few cyanobacterial proteins have been isolated and tested for kinetic data, many of the Kms compared are from other species. 4ABH has a Km of 20.4µM for p-amino benzoate(PABA), while the folate enzyme folp1 has a Km of 0.37µM[5,6]. Assuming the rate and quantity are the same, the folate enzyme will be fully saturated at a much lesser concentration of PABA than 4ABH. You can assume that the Km ratio of 20.4:0.37 (or 55:1) will represent how many moles of PABA goes to folate versus acetaminophen. For the tryptophan pathway, 4ABH activity for antranilate is assayed as having a rate of 24% of PABA, equal to 34µM for anthranilate. This compares to TrpD's Km for chorismate of 40nM, meaning we'd have 850:1 tryptophan to acetaminophen. Since these ratios are based on differnt enzymes than our organism, we will keep this enzyme competition in mind as a potential problem while using 33% precursor conversion rate for simplicity.
Assumptions
- We assume that our genes were designed, inserted, and translated successfully and in reasonable quantities.
- We assume Km values for Arthrospira platensis are the same as organisms' enzyme Kms. Ortholog sequences were BLAST compared in each case, resulting in a range of similarities between 52% and 63% alignment, suggesting our organism's Km might be significantly different.
- We assume that enzyme rate and quantity is the same for each 4ABH, folp1, and TrpD, else we could not compare Km ratios and calculate approximate the Km of 4ABH for anthranilate.
Acetaminophen Conclusion
These numbers show that there will probably be enough precursor to produce a useful, detectable quantity of acetaminophen. Based on literature and sequence estimates of aromatic amino acids, we can assume there would be at least that many moles of chorismate from which our added pathway pushes towards acetaminophen. The three calculations above can be averaged to finally predict 8.26mg ± 2.77mg acetaminophen per gram of Arthrospira platensis biomass.
We used different tryptophan estimates to reach several different predictions for acetaminophen, averaging 8.26mg ± 2.77mg acetaminophen per gram of Arthrospira platensis biomass or 8.26µg per mL. This would be significantly above the limit of detection for our HPLC, at 50ng per ml, and serve as a starting point for optimizing production. This means that one 325mg dose of acetaminophen could be obtained in ~39g of biomass, meaning a 12 by 3 feet round pool could produce enough acetaminophen for more than 200 people every 10 days. While 39 grams isn't an ideal amount of medicine to consume, it does show that Arthrospira platensis has significant potential as a molecular factory for acetaminophen.
VITAMIN B12

The quantity of DMB B12 produced depends on a successful production and integration of the active B12 lower ligand, 5,6-dimethyl-benzimidazole (5,6-DMB). For phytoplankton in the wild, cobalt is often the limiting factor for growth and production of B12[11,12], while B12 production is limited by growth need in optimal media[12]. With ssuE and bluB genes inserted and regulated using a strong PrtC promoter, the activating lower ligand 5,6-DMB will be created in abundance[13]. Synechococcus and Arthrospira platensis both have CobS, bluB, and pGam genes that code for proteins which bind 5,6-DMB to the cobalt[14,15]. If these proteins work as well as in their origin organism, then assays report that 5,6-DMB has at least 100 times higher affinity for cobalt than the B12 analog ligand, adenine[16,17], meaning the DMB B12 to B12 analog ratio would be 100:1. Published HPLC results show that that Arthrospira platensis produces between 1.5-2.5µg B12 analogs per gram dry weight[18].
$$\frac{2.5µg\ B_{12}\ analog}{1g\ drymass} * \frac{100\ DMB\ bindings}{101\ DMB+adenine\ binding} = \frac{2.47µg\ DMB\ B_{12}}{1g\ biomass}$$
An additional paper assayed Synechococcus elongatus sp WH 7803 at 10-18 moles per cell[16], and at a reported density 109 cells per liter (about a gram)[16,17], Synechococcus would produce 1.3µg B12 per liter dry mass. Another older paper used microbiological assays along with TLC and HPLC, finding maximums of 2.4, 1.47, and 1.27µg B12 per gram dry mass. Averaging the 6 data points and multiplying by a 100:1 conversion ratio results in a predicted production of 1.74 ± 0.23µg DMB-B12 per gram of Arthrospira platensis drymass, meaning the USDA’s recommended daily value of 6µg could be obtained in one 3.5 gram serving.
Assumptions
- Gene inserts will be expressed, converting riboflavin-5′-phosphate to 5,6-DMB in excess[13].
- The bluB/CobS protein complex in Arthrospira platensis will attach 5,6-DMB to cobalt at rates similar to those assayed in Propionibacterium freudenreichii[14,15].
- Cobalt will be provided in excess of 0.3mM according the BG-11 recipe, ensuring maximum precursor availability[16,17]
- Synechococcus 7942, 7803 and Arthrospira platensis will have similar rates of B12 production.
Future B12 projects might use chemo-trophic bacteria such as Methanosarcina barkeri which produces more than 1000 times more B12 and could be converted to active form using a similar bioengineering proccess[21]. One exceptional use of cyanobacterial B12 is growing cyanobacteria in the water used to grow rice, increasing the carbon fixation, nitrogen fixation, and bioenriching the rice with B12[18].
BIOMASS

To understand the production capacity of our organisms, we aggregated growth data from published papers[22,23,24] and our own daily optical density growth data. Using carrying-capacity-limited logistic growth curves to fit our data to an equation, we modelled dried biomass with respect to time. We also added additional dependent variables of temperature and light intensity to our equation so that we can better understand how minimalist growth conditions might affect the maximum culture density and the maximum growth rate.
Light Intensity: μE m-2 s-1
Temperature: ℃




