| Line 324: | Line 324: | ||
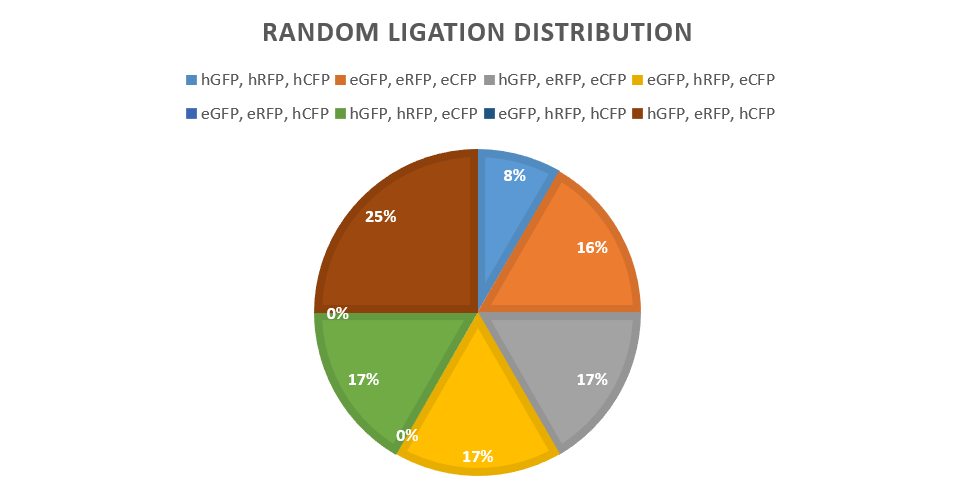
<p> The random ligation of different promoter-protein bricks is an experiment to test the viability of a brownian motion driven random ligation process. This random ligation is the basis for the unpredictability of a large scale key design process. The experiment hoped to produce a random mixture of fluorescent protein expression levels. This random mixture of multiple proteins in a single vector would then show the viability of the Key.coli restriction enzyme-ligation process for achieving unpredictability for keys.</p> | <p> The random ligation of different promoter-protein bricks is an experiment to test the viability of a brownian motion driven random ligation process. This random ligation is the basis for the unpredictability of a large scale key design process. The experiment hoped to produce a random mixture of fluorescent protein expression levels. This random mixture of multiple proteins in a single vector would then show the viability of the Key.coli restriction enzyme-ligation process for achieving unpredictability for keys.</p> | ||
<h1>How</h1> | <h1>How</h1> | ||
| − | <p> The highest and lowest performing promoters were chosen to give the most easily visible result. Promoter E and Promoter 4. Promoter 4 gives a high expression of fluorescent proteins, as shown by our promoter library findings. The promoters were then attached to each reporter protein CFP, RFP and GFP to form six "brick" variants. After amplification of the bricks produced, seven products combinations were ligated to a low copy backbone | + | <p> The highest and lowest performing promoters were chosen to give the most easily visible result. Promoter E and Promoter 4. Promoter 4 gives a high expression of fluorescent proteins, as shown by our promoter library findings. The promoters were then attached to each reporter protein CFP, RFP and GFP via BSAI digestion and ligation (creating no scar sites) along with a terminator to form six "brick" variants. After amplification of the bricks produced via PCR, seven products combinations were ligated to a low copy backbone in the pattern of red, green and then blue (fluorescent protein) consistently through controlled use of the DCBA digestion site. In addition to a set of "random ligations". Each ligation has only one ligation slot (due to availability of restriction cut sites) per reporter type/colour, leaving random chance to produce a combination of all the possible variants in the random ligations where multiple promoters are available.</p> |
| + | <p> e= empty promoter, h= high expression promoter</p> | ||
<center><img src="https://static.igem.org/mediawiki/2017/a/a1/T--UNOTT--brickstitching.jpeg"></center> | <center><img src="https://static.igem.org/mediawiki/2017/a/a1/T--UNOTT--brickstitching.jpeg"></center> | ||
<p> These bricks were ligated with the backbone as follows: </p> | <p> These bricks were ligated with the backbone as follows: </p> | ||
Revision as of 18:55, 1 November 2017

EXPERIMENTS: