| Line 336: | Line 336: | ||
<div class="whiteboxmargin"> | <div class="whiteboxmargin"> | ||
<h1>Why</h1> | <h1>Why</h1> | ||
| − | <p> The random ligation of different promoter-protein bricks is an experiment to test the viability of a brownian motion driven random ligation process. This random ligation is the basis for the unpredictability of a large scale key design process. The experiment hoped to produce a random mixture of fluorescent protein expression levels. This random mixture of multiple proteins in a single vector would then show the viability of the Key.coli restriction enzyme-ligation process for achieving unpredictability for keys.</p> | + | <p> The random ligation of different promoter-protein bricks is an experiment to test the viability of a brownian motion driven random ligation process. This random ligation is the basis for the unpredictability of a large scale key design process. The experiment hoped to produce a random mixture of fluorescent protein expression levels. This random mixture of multiple proteins in a single vector would then show the viability of the <i>Key. coli</i> restriction enzyme-ligation process for achieving unpredictability for keys.</p> |
<h1>How</h1> | <h1>How</h1> | ||
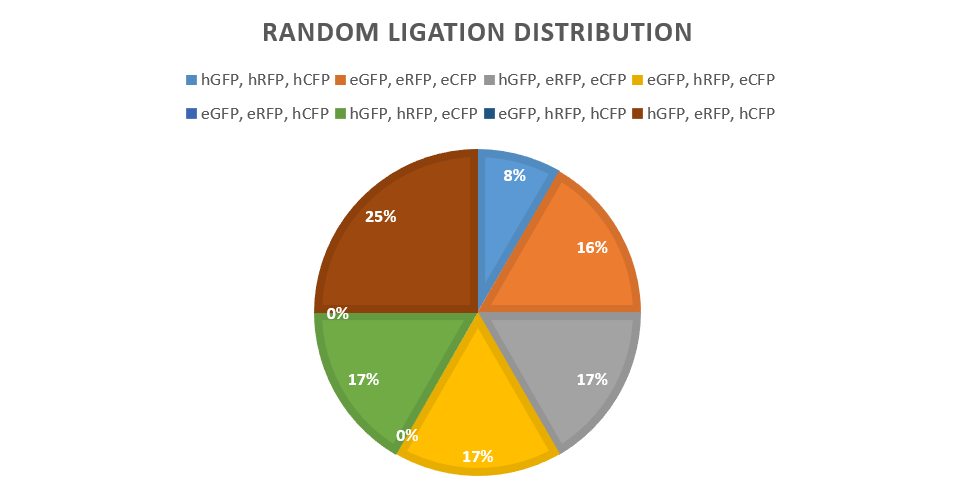
<p> The strongest and weakest promoters were chosen to give the most easily visible result, promoters E and 4. Promoter 4 gives a high expression of fluorescent proteins, as shown by our promoter library findings. The promoters were then attached to each reporter protein CFP, RFP and GFP via BsaI digestion and ligation along with a terminator to form six "brick" variants. After amplification of the bricks produced via PCR, seven products combinations were ligated to a low copy backbone in the pattern of red, green and blue fluorescent proteins, consistently through the controlled use of the DCBA digestion site. Each ligation has only one ligation slot (due to availability of restriction cut sites) per reporter colour, leaving random chance to produce a combination of all the possible variants in the random ligations where multiple promoters are available.</p> | <p> The strongest and weakest promoters were chosen to give the most easily visible result, promoters E and 4. Promoter 4 gives a high expression of fluorescent proteins, as shown by our promoter library findings. The promoters were then attached to each reporter protein CFP, RFP and GFP via BsaI digestion and ligation along with a terminator to form six "brick" variants. After amplification of the bricks produced via PCR, seven products combinations were ligated to a low copy backbone in the pattern of red, green and blue fluorescent proteins, consistently through the controlled use of the DCBA digestion site. Each ligation has only one ligation slot (due to availability of restriction cut sites) per reporter colour, leaving random chance to produce a combination of all the possible variants in the random ligations where multiple promoters are available.</p> | ||
Revision as of 01:46, 2 November 2017

EXPERIMENTS: