| Line 32: | Line 32: | ||
<div class="pop"> | <div class="pop"> | ||
<a href="https://static.igem.org/mediawiki/2017/c/c6/T--Hong_Kong_UCCKE--300sequence.jpg" title="300 sequencing result"> | <a href="https://static.igem.org/mediawiki/2017/c/c6/T--Hong_Kong_UCCKE--300sequence.jpg" title="300 sequencing result"> | ||
| − | <img src="https://static.igem.org/mediawiki/2017/c/c6/T--Hong_Kong_UCCKE--300sequence.jpg" alt="300 sequencing resultl"style="width:100%; max-width: | + | <img src="https://static.igem.org/mediawiki/2017/c/c6/T--Hong_Kong_UCCKE--300sequence.jpg" alt="300 sequencing resultl"style="width:100%; max-width:800px;"> |
</a> | </a> | ||
<br><span class="imgcaption">Sequencing result of BBa_K2197300</span> | <br><span class="imgcaption">Sequencing result of BBa_K2197300</span> | ||
| Line 52: | Line 52: | ||
<div class="row"><div class="col-md-12"> | <div class="row"><div class="col-md-12"> | ||
<p><font size="4">Conclusion</font></p> | <p><font size="4">Conclusion</font></p> | ||
| − | <p style="text-align:left !important;"> From the results above, we can say we successfully clone the DNA that IDT sent us into the cell. However, as what we have mentioned in the experiment page, we found that the | + | <p style="text-align:left !important;"> From the results above, we can say we successfully clone the DNA that IDT sent us into the cell. However, as what we have mentioned in the experiment page, we found that the cells cannot express GFP. Then, we aligned the sequence registered in IGEM with the one that IDT send us, we found that the sequence containing 'tactagag' is missing.</p> |
</div> | </div> | ||
</div> | </div> | ||
| Line 76: | Line 76: | ||
<p style="text-align:left !important;">After mini prep, we use the same restriction enzyme EcoR1 and Pst1 to do restriction digestion and gel electrophoresis. As seen, the size of K2197400 (2214 bp) and the backbone pSB1C3 (2070 bp)is almost the same, similar to our predicted result.</p> | <p style="text-align:left !important;">After mini prep, we use the same restriction enzyme EcoR1 and Pst1 to do restriction digestion and gel electrophoresis. As seen, the size of K2197400 (2214 bp) and the backbone pSB1C3 (2070 bp)is almost the same, similar to our predicted result.</p> | ||
| − | <div class="pop"> | + | |
| − | <a href="https://static.igem.org/mediawiki/2017/5/54/T--Hong_Kong_UCCKE--21asdfghj2.jpg" title="Image of gel electrophoresis of | + | |
| − | <img src="https://static.igem.org/mediawiki/2017/5/54/T--Hong_Kong_UCCKE--21asdfghj2.jpg | + | |
| − | </a> | + | <div class="row pop"> |
| + | <div class="col-xs-6 col-sm-5 col-md-4"> | ||
| + | <a href="https://static.igem.org/mediawiki/2017/5/54/T--Hong_Kong_UCCKE--21asdfghj2.jpg" title="Image of gel electrophoresis of 500"><img src="https://static.igem.org/mediawiki/2017/5/54/T--Hong_Kong_UCCKE--21asdfghj2.jpg" style="width:100%;"/></a> | ||
<br><span class="imgcaption">Image of gel electrophoresis of 400a+b and 500</span> | <br><span class="imgcaption">Image of gel electrophoresis of 400a+b and 500</span> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | |||
| + | <div class="col-xs-6 col-sm-5 col-md-4"> | ||
| + | <a href="https://static.igem.org/mediawiki/2017/2/2b/T--Hong_Kong_UCCKE--theoracpic400.jpg" title="Image of gel electrophoresis of 500"><img src="https://static.igem.org/mediawiki/2017/2/2b/T--Hong_Kong_UCCKE--theoracpic400.jpg" style="width:100%;"/></a> | ||
| + | <br><span class="imgcaption">Image of theoretical gel electrophoresis of 400 from genome compiler</span> | ||
| + | </div> | ||
| + | </div> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| Line 103: | Line 107: | ||
<div class="pop"> | <div class="pop"> | ||
| − | <a href="https://static.igem.org/mediawiki/2017/c/c1/T--Hong_Kong_UCCKE--gelsequence400.png" style="width:100%; max-width:300px;"/><img src="https://static.igem.org/mediawiki/2017/c/c1/T--Hong_Kong_UCCKE--gelsequence400.png" style="width:100%; max-width: | + | <a href="https://static.igem.org/mediawiki/2017/c/c1/T--Hong_Kong_UCCKE--gelsequence400.png" style="width:100%; max-width:300px;"/><img src="https://static.igem.org/mediawiki/2017/c/c1/T--Hong_Kong_UCCKE--gelsequence400.png" style="width:100%; max-width:800px;"/></a> |
<br><span class="imgcaption">Sequencing Result of BBa_K2197400</span> | <br><span class="imgcaption">Sequencing Result of BBa_K2197400</span> | ||
<br> | <br> | ||
Revision as of 15:46, 1 November 2017
After we have successfully cloned our plasmids into the E.coli, we did miniprep to obtain purified plasmid DNA. Then, we did restriction map and have sent the samples to BGI for sequencing. The results are shown below.
BBa_K2197300
Restriction Map
After mini prep, we use the same restriction enzyme EcoR1 and Pst1 to do restriction digestion and gel electrophoresis. However, the size of K2197300 and the backbone pSB1C3 is almost the same, thus we cannot prove by the Gel photo.
Sequencing Result
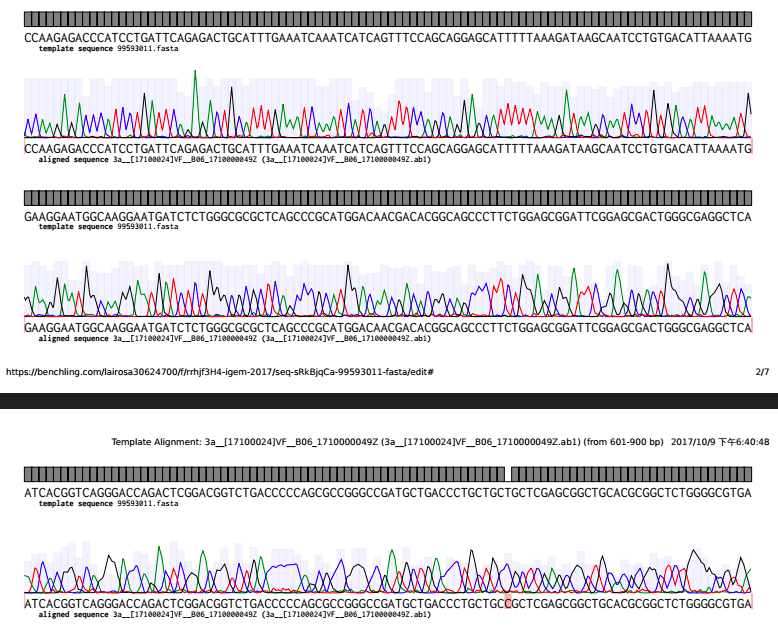
With the help of CUHK, we sent our plasmid to BGI for sequencing. We aligned the sequence that we ordered from IDT(As the template sequence 99593011 below) with the sequencing result received from BGI(as the aligned sequence below). And here is our analysis.
The black area indicates that the two sequence are the same which those red strip represent that there're some mutations.
Conclusion
From the results above, we can say we successfully clone the DNA that IDT sent us into the cell. However, as what we have mentioned in the experiment page, we found that the cells cannot express GFP. Then, we aligned the sequence registered in IGEM with the one that IDT send us, we found that the sequence containing 'tactagag' is missing.
BBa_K2197400
Restriction Map
After mini prep, we use the same restriction enzyme EcoR1 and Pst1 to do restriction digestion and gel electrophoresis. As seen, the size of K2197400 (2214 bp) and the backbone pSB1C3 (2070 bp)is almost the same, similar to our predicted result.
Sequencing Result
With the help of CUHK, we sent our plasmid to BGI for sequencing. We aligned the sequence that we ordered from IDT(As the template sequence 163014570 below) with the sequencing result received from BGI(as the aligned sequence below). And here is our analysis.
The black area indicates that the two sequence are the same which those red strip represent that there're some mutations.
Conclusion
From the results above, we can say we successfully clone the plasmid DNA into the cell.
BBa_K2197500
Restriction Map
Also using the same restriction enzyme EcoR1 and Pst1 to do restriction digestion and gel electrophoresis. The size of K2197500 (1668 bp) and the backbone pSB1C3 (2070 bp).
Sequencing Result
We sent our plasmid to BGI for sequencing. We aligned the sequence that we ordered from IDT(As the template sequence 163014569 below) with the sequencing result received from BGI(as the aligned sequence below). And here is our analysis.
The black area indicates that the two sequence are the same which those red strip represent that there're some mutations.

Sequencing Result of BBa-K2197500
Conclusion
From the results above, we can say we successfully clone the plasmid DNA into the cell.
BBa_K2197502
Sequencing Result
We sent our plasmid to BGI for sequencing. We aligned the sequence that we ordered from IDT(As the template sequence 163014567 below) with the sequencing result received from BGI(as the aligned sequence below). And here is our analysis.
The black area indicates that the two sequence are the same which those red strip represent that there're some mutations.
Conclusion
From the results above, we can say we successfully clone the plasmid DNA into the cell.