

Successfully digested, ligated, and transformed rfbE into pSB1C3
The objective of this experiment was to digest and ligate the rfbE BioBrick BBa_K2495001 into the pSB1C3 linearized backbone for DNA submission and characterization. The rfbE gene was obtained in the BioBrick format from IDT. A Modified restriction double digest and ligation protocol was used and can be found on our protocol page. We confirmed this by running PCR and a gel.
Optimized the LAMP reaction to 20 minutes without lysis or centrifugation
The optimal conditions for cell lysis were previously investigated using E. coli DH5α transformed with pGLO plasmid (see biology lab notebook. The optimal cell lysis conditions for use with LAMP were tested using rfbE transformed into E. coli.
E. coli DH5α was transformed with rfbE using the pJET cloning vector. Additionally, naturally occurring E. coli was isolated from a sample of beef that was left at room temperature overnight. Miniprep (Qiagen) was used to isolated the plasmid DNA of these cells for the following experiments.
To show that we have successfully cloned rfbE into the pJET vector and to show the specificity of our PCR primers (BBa_K2495019,BBa_K2495020) targeting rfbE that we designed using PrimerExplorer V5, we ran PCR of our samples below according to the following program:
- Initial denaturation at 95 ºC for 5 min
- 30 cycles of denaturation at 94 ºC for 30 s, annealing at 50 ºC for 45 s
- Final extension at 72 ºC for 1.5 min
- Hold at 4 ºC
LAMP was run according to the instructions of the manufacturer (OptiGene) using primers targeted at rfbE designed with PrimerExplorer V5. The experimental conditions were:
- rfbE gene fragment (IDT) - positive control
- Plasmid DNA of rfbE in DH5α - negative control
- Plasmid DNA of rfbE in DH5α treated with 10 minutes of 95˚C heat lysis - negative control
- Plasmid DNA of rfbE in DH5α
- rfbE in DH5α suspended in TE buffer and treated with 10 minutes of 95˚C heat lysis
- rfbE in DH5α suspended in TE buffer (without heat treatment)
- Plasmid DNA of beef E. coli
- Plasmid DNA of beef E. coli treated with 10 minutes of 95˚C heat lysis
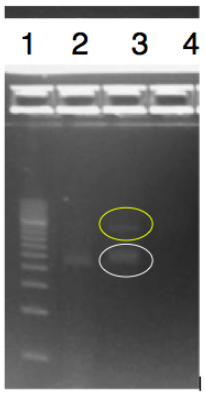
The PCR reaction showed a clear band at 1000-bp (circled in black), while LAMP showed the typical smear of amplification. Both PCR and LAMP reactions did not show any banding or smearing in the DH5α or beef E. coli results, indicating that both techniques are specific to our gene of interest. However, comparison of the heat versus no heat lysis variables (circled in red) show that LAMP is able to amplify our gene of interest in the absence of the heat lysis step. This indicates that the LAMP technique is much more sensitive than PCR.
Due to the results of this experiment, we envision that it will not be necessary to include a heat lysis step in our final version of the detection process for Shiga-toxigenic bacteria.


Sensitive to 106 cells/mL
To determine the limit of detection of our LAMP reactions, we ran it on serial dilutions of inoculated broth and monitored the progress using the Applied Biosystems SteponePlus Real-Time PCR System (Thermo Fisher, MA). Inoculated broth was diluted and run according to the optimized protocol described in our biology lab notebook. The dilutions ranged from 108 cells/mL to 101 cells/mL. Six serial dilutions of a previously miniprepped sample were used as positive controls. The negative control was TE buffer. The reaction was run on a total volume of 12.5µl. Duplicates were averaged and the fluorescence was plotted against time using Excel.
The results corresponded with a subsequent gel on the amplified products. The band intensity of the gels were in agreement with the relative fluorescence intensities visualized during the reaction.


While samples of lower concentrations were run, we could not draw any conclusive evidence from these as they did not differ significantly from the fluorescence intensity of the control. The fluorescence intensity of the broth with 108 cells/mL was significantly higher than the highest concentration of miniprepped sample (66 ng/mL). This does not correspond to the gel, in which lane 2 shows a stronger band than in lane 5. This may be due to the high concentration of cell debris that may have inhibited the reaction. Concentrations between 107 cells/mL and 106 cells/mL have decreasing fluorescence intensity and we are confident that our detection is accurate to this bacterial concentration.
We have found that using a larger reaction volume (25µl) does increase our sensitivity significantly, but due to shipping difficulties we were unable to obtain enough MasterMix to continue testing with a 25 µl reaction volume. Instead, subsequent tests were tested with 12.5 µl volume.
Based on these results, we determined that our limit of detection is 106 cells/mL.


The reaction products were also visualized under blue light and UV light at 302 nm. We can clearly visualize a gradient in the fluorescence from highest to lowest concentrations of DNA. All samples are clearly more fluorescent than the negative control.

Constructed a PDMS chip to house the reaction

Each chip design was tested with the following conditions and requirements:
- Able to let a volume of 25 ul of sample to flow into the necessary reaction chamber.
- Mix sufficiently well with the reagents (Simulated with water and dye for experimentation).
- Prevent any leakage of fluid either via gaps or evaporation.
After testing numerous chip prototypes, we designed the optimized chip design that shows both successful fluid flow and thermal performance when subjected to 65˚C heating for LAMP reactions. A 5-well design was the result which prevented any fluid loss due to leakage and optimum mixing between the sample and LAMP reagents. No valving was necessary and the entire chip is 40 mm x 25 mm, making it much smaller and thus cheaper than previous designs.
Each well can hold up to a of 50 µl, which is more than enough to contain both liquid reagents and sample. Thus, our design is not only limited to just lyophilized reagents. Previous designs only showed successful fluid flow but were not able to prevent condensate from escaping. The current design solves this by covering the wells with a hydrophobic, transparent material (optical adhesive) that does not interfere with fluorescence results.
Designed a heating circuit
 When developing the heating method, we found that it needed to satisfy the following properties:
When developing the heating method, we found that it needed to satisfy the following properties:
- Sustain a temperature of 65˚C for the reaction
- Contain feedback control to maintain the temperature
- Affordable
Between Peltier Cooler Modules, ITO heaters and hand warmers, we chose the Peltier because we found that it could reach the desired temperature, was easy-to-use and customize, and was reasonably priced compared to the ITO heater. The Peltier can be used with 5-7 V voltage input and 1-2 A current input. This property was ideal, considering that most portable power banks have a DC output of 5 V and 2.1 A. Hence, the heater was developed such that a portable power bank can act as the power supply.
To control the Peltier Cooler Module, we used the tmp36 temperature sensor because it is cheap and easy-to-use. Additionally, an Arduino nano was used as the microchip controller due to its simplicity, a compact size, and its ability to do a proportional-integral-derivative (PID) control using tmp36. Finally, an N-Mosfet transistor was used to act as a switch control for the Peltier heater.
An external power supply was added using a power adapter that allows for 6 V and 1.5 A. The Arduino nano was powered by Laptop USB, which only allows 500 mA of current.The tmp36 uses the 5 V voltage as a reference to measure the temperature on the Peltier heating plate. This sensor controls the N-Mosfet transistor that works with a low-side switch setup, allowing the current to flow whenever the temperature of the Peltier registers below 65˚C.
The prototype of our STEC-detecting device was constructed using a PDMS chip containing 5 wells of 3 mm of diameter sealed with PCR-compatible optical adhesive film. The 65 ºC temperature was achieved using a Peltier Modular Cooling system with a 6 Volts, 1.5 Amperes external power supply adapter. For more information on the design of the chip and the Peltier system used, please refer to the lab notebook.
All the wells were loaded with 5 μL of Isothermal Master Mix (OptiGene), 1.25 μL of 10X SYBR Green (BioRad), 0.25 μL of F3 primer (BBa_K2495014), 0.25 μL of B3 primer (BBa_K2495015), 1 μL of FIP primer (BBa_K2495016), and 1 μL of BIP primer (BBa_K2495017). All primers were procured from Sigma Aldrich.
Figure 1 shows the wells under blue light after running the LAMP reaction. It is possible to observe that the fluorescence of wells 3 through 5 is as strong as the positive control, and all of the wells show a stronger fluorescence than the negative control.

A time lapse of the reaction is available in video format.
In future trials, the use of microfluidics channels will be investigated to provide a more user-friendly method to load the samples at once. The device will also be tested with a broader range of bacteria concentrations and on different pathogens in the near future.

Chen, et al. (2017) developed a similar device which required the use of centrifugation and heat lysis. Their device was estimated to cost $550 USD, which is considerably more expensive and requires more laboratory techniques than the method we have developed.




