| Line 16: | Line 16: | ||
Thanks to these surveys, we noticed that a product administered by injection was harder to accept by farmers because it was much harder to implement on large farms. | Thanks to these surveys, we noticed that a product administered by injection was harder to accept by farmers because it was much harder to implement on large farms. | ||
Consequently, given that it was not a priority for these stakeholders, our Hardware team stopped working on an automated injection system and concentrated on detection. | Consequently, given that it was not a priority for these stakeholders, our Hardware team stopped working on an automated injection system and concentrated on detection. | ||
| − | We also decided to test the delivery system of our treatment | + | We also decided to test the delivery system of our treatment, to see if it would penetrate plants and be able to have an action against [[Team:Aix-Marseille/Xylella_fastidiosa|''Xylella fastidiosa'']]. |
Our experiments, guided by the stakeholder survey, and with out [[Team:Aix-Marseille/M13_test|experiments on ''Arabidopsis thaliana'']] we tested the efficiency of different application methods. | Our experiments, guided by the stakeholder survey, and with out [[Team:Aix-Marseille/M13_test|experiments on ''Arabidopsis thaliana'']] we tested the efficiency of different application methods. | ||
| Line 23: | Line 23: | ||
This also drew our attention to the legislation related to the use of phages. | This also drew our attention to the legislation related to the use of phages. | ||
We concluded that a product containing genetically modified phages would be very hard to market because of it would be considered dangerous. | We concluded that a product containing genetically modified phages would be very hard to market because of it would be considered dangerous. | ||
| − | Both of these elements lead us to redesign our | + | Both of these elements lead us to redesign our project. |
We met [[Team:Aix-Marseille/HP/Interviews#Mireille_ANSALDI|Ms Mireille Ansaldi]], the PI of the phage cycle and bacteria metabolism team in the LCB, to find another solution for using a phage. | We met [[Team:Aix-Marseille/HP/Interviews#Mireille_ANSALDI|Ms Mireille Ansaldi]], the PI of the phage cycle and bacteria metabolism team in the LCB, to find another solution for using a phage. | ||
| − | We decided at this point to use phage-like particles (instead of a phage) as their inability to self replicate would also make them more acceptable. | + | We decided at this point to use phage-like particles (instead of a phage) as their inability to self-replicate would also make them more acceptable. |
Finally, in order to get advice on the relevance of our project we contacted [[Team:Aix-Marseille/HP/Interviews#Marie-Agn.C3.A8s_JACQUES|Ms Marie-Agnès Jacques]], a [[Team:Aix-Marseille/Xylella_fastidiosa|''X. fastidiosa'']] specialist from the Institut National de la Recherche Agronomique (INRA). She warned us that we would be defined by the legislation as a GMO product. We then began a complete study of [[Team:Aix-Marseille/Legislation|French and European law]], thanks to a collaboration with the [https://2017.igem.org/Team:Evry_Paris-Saclay|Evry Paris-Saclay] team. This allowed us to see if our product could be categorized other than GMOs and if we will be able to sell it in France and in Europe. We wanted to make sure that our product, especially our phage-like particle as it is a GMO, was environmentally friendly and didn't pollute the soil or harm plant. To do so, we [[Team:Aix-Marseille/M13_test|tested]] our phages in various conditions and compared our results to legal rates established by French and European institutions. | Finally, in order to get advice on the relevance of our project we contacted [[Team:Aix-Marseille/HP/Interviews#Marie-Agn.C3.A8s_JACQUES|Ms Marie-Agnès Jacques]], a [[Team:Aix-Marseille/Xylella_fastidiosa|''X. fastidiosa'']] specialist from the Institut National de la Recherche Agronomique (INRA). She warned us that we would be defined by the legislation as a GMO product. We then began a complete study of [[Team:Aix-Marseille/Legislation|French and European law]], thanks to a collaboration with the [https://2017.igem.org/Team:Evry_Paris-Saclay|Evry Paris-Saclay] team. This allowed us to see if our product could be categorized other than GMOs and if we will be able to sell it in France and in Europe. We wanted to make sure that our product, especially our phage-like particle as it is a GMO, was environmentally friendly and didn't pollute the soil or harm plant. To do so, we [[Team:Aix-Marseille/M13_test|tested]] our phages in various conditions and compared our results to legal rates established by French and European institutions. | ||
Revision as of 00:25, 2 November 2017
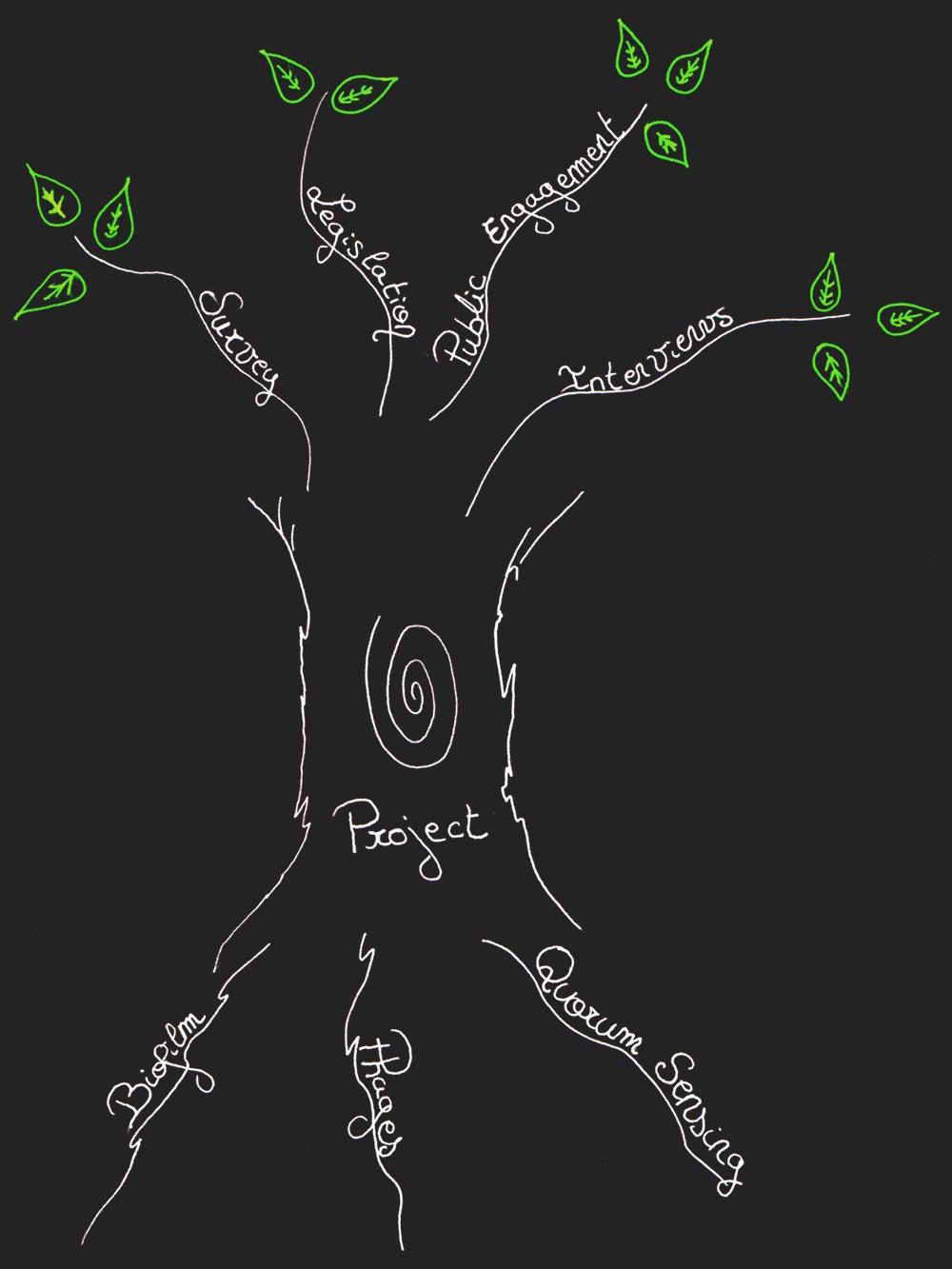
Integrated Human Practices
We have thought about the Human Practices since the very beginning of KILL XYL. We had defined the actors involved and how they impacted our scientific approach. This way, our project could be as adapted as possible to the real needs of society.
Then, as our project advanced and the technical aspects were defined, we worked more on the practical aspects of Human Practices.
First and foremost, safety is crucial in a lab. As we work in synthetic biology, we had to consider the safety level of each part and organisms we used. We made certain to work at all times in the best conditions. Experiments with P2 level organisms were performed by trained advisors.
To integrate farmer’s opinions, we contacted them and made a survey, first via the web and then in person, during an agricultural fair. This approach helped us to figure out their needs and their thoughts about Xylella fastidiosa, so we could work accordingly.
Thanks to these surveys, we noticed that a product administered by injection was harder to accept by farmers because it was much harder to implement on large farms. Consequently, given that it was not a priority for these stakeholders, our Hardware team stopped working on an automated injection system and concentrated on detection. We also decided to test the delivery system of our treatment, to see if it would penetrate plants and be able to have an action against Xylella fastidiosa. Our experiments, guided by the stakeholder survey, and with out experiments on Arabidopsis thaliana we tested the efficiency of different application methods.
One of the most important changes in our project design caused, at least in part, by farmers' opinion was moving away from phages. We understood very clearly that they did not feel comfortable with the idea of spreading phages in Nature. This also drew our attention to the legislation related to the use of phages. We concluded that a product containing genetically modified phages would be very hard to market because of it would be considered dangerous. Both of these elements lead us to redesign our project. We met Ms Mireille Ansaldi, the PI of the phage cycle and bacteria metabolism team in the LCB, to find another solution for using a phage. We decided at this point to use phage-like particles (instead of a phage) as their inability to self-replicate would also make them more acceptable.
Finally, in order to get advice on the relevance of our project we contacted Ms Marie-Agnès Jacques, a X. fastidiosa specialist from the Institut National de la Recherche Agronomique (INRA). She warned us that we would be defined by the legislation as a GMO product. We then began a complete study of French and European law, thanks to a collaboration with the Paris-Saclay team. This allowed us to see if our product could be categorized other than GMOs and if we will be able to sell it in France and in Europe. We wanted to make sure that our product, especially our phage-like particle as it is a GMO, was environmentally friendly and didn't pollute the soil or harm plant. To do so, we tested our phages in various conditions and compared our results to legal rates established by French and European institutions.
To sum up, our project grew permanently, intertwined with the human practices. Our various investigations about human practices called our project into question all the time. We changed our mind several times about different points and initiated additional experiments further to our research in Human Practices.