Lactobacillus plantarum
Lactobacillus plantarum is a gram-positive lactic acid producing bacteria, so it requires a different growth media than we typically use in our lab. In 1954, Briggs agar was developed (5). This media was designed for lactobacilli, but was not sufficient for many species, including Lactobacillus plantarum , so a different non-selective media for general lactobacilli was developed in 1960 by Man, Rogosa and Sharpe and named MRS(6). We have exclusively grown our Lactobacillus plantarum on MRS media. Further, we grew Lactobacillus plantarum in a CO2 incubator as referenced in most literature we studied (1)(2)(3). The metabolic pathways in the bacteria alters when grown aerobically to produce excess acetate (7) and less lactic acid. Because we intend to utilize this bacteria in a fermentable food, a change in this metabolic pathway would not benefit our ultimate goal.
- MRS broth was prepared using 55g of Difco MRS broth powder in 1L of distilled water. The solution was then autoclaved and stored in a 4 degree refrigerator along with the Difco MRS powder.
- MRS agar was prepared by mixing 55g of Difco MRS broth powder and 15g of agar flakes in 1 L of water. The solution was then autoclaved and stored in a 4 degree refrigerator along with the Difco MRS powder.
Once we could successfully grow our chosen bacteria, we needed to transform the gram positive, Lactobacillus plantarum, with pMSP3535. In order to do this, we identified and worked with a different protocol than we had ever used in our lab. We attempted several protocols, including Landete 2014 (2) and Speer 2012 (1). However, we found success using a variation of the Welker protocol(3). Welker et al. transformed multiple strains of Lactobacillus casei using varying reagents and yielded different efficiencies between each strain of the species with each variation (3).
The Texas Tech iGEM team helped us by testing the Speer protocol after we had attempted three procedures and hadn't successfully transformed. They were not able to successfully transform using the Speer protocol which suggests that the procedure was either too simplified for researchers who have never transformed gram-positive bacteria, or was not compatible with Lactobacillus plantarum.
Materials Needed
- MRS liquid media
- L. plantarum cells
- Glass culture tube
- CO2 incubator
- MRS liquid media, containing 0.5 M sucrose
- Spectrophotometer
- Centrifuge
- Vortex
- 50 mL Falcon conical tubes (2)
- 30% PEG 8000
Preparation of Recipient Cell Stocks
- Inoculate 10-25 mL of -80 ࠷ L. Plantarum stocks in MRS broth at 37 ࠷ CO2 incubator, overnight without shaking.
- Subculture by incubating culture in a 200 mL of prewarmed MRS broth. Add culture until OD600 is 0.1.
- Subcultures should be grown to an OD600 of 0.6 (4-8 hours).
- For glycine supplementation, the MRS broth should contain 0.5%-1.0% glycine.
- For NaCl supplementation, inoculate subcultures with 0.9M of NaCl.
- Harvest cells from the subculture by centrifugation at 4 ࠷ for 10 min. at 7000 rpm after inoculation.
- Rinse the cells in 200 mL of sterile, distilled water (~4 ࠷) and centrifuge again.
- Resuspend the pellet in 2-3 mL of cold, sterile, distilled water and aliquot 1 mL volumes to 1.5 mL microcentrifuge tubes.
- Centrifuge aliquots at 15,000 rpm for ~90 seconds and remove the supernatant.
- Rinse the cells twice with 1 mL of cold, distilled, sterile water. Then rinse with 1 mL of cold, sterile 30% PEG-8000 solution. Remove the supernatant.
- Suspend cells in 0.5-0.6 mL of 30% PEG for storage at -80 ࠷. These should retain viability for up to 2 years.
Treatment of Cells Prior to Electroporation
- Thaw on Ice.
- Suspend cells in 900μL of cold, sterile, distilled water for 30 min.
- Treat with 100mM lithium acetate and 10mM DTT solution for 30min.
- After pretreatment, the cells were pelleted for 2-3 minutes in a microcentrifuge, washed once in 1 mL of cold, sterile, 30% PEG solution and suspended in 0.5-0.6 mL cold, sterile PEG solution for electroporation.
Electroporation
- Isolate intended vector (Lactobacillus plantarum experiment as of 09/06/17 has been using pMSP3535).
- 200 ng/transformation of plasmid DNA is mixed with 100μL of cell suspension and transferred to a prechilled electroporation cuvette with a 0.2 cm gap.
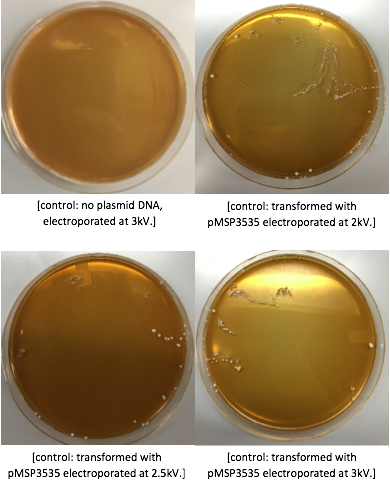
- Pulse at 12.5kV (From Literature: Bio-rad gene pulser with capacitance setting at 25μF and resistances of 200 or 400 Ω and voltage settings of 1500, 2000 or 2500 V (7.5, 10 or 12.5 kV cm-1).
- Quickly recover cells by adding 900μL of recovery medium (0.5 M sucrose in MRS broth) to the cuvette then transfer the cells to a sterile microcentrifuge tube for incubation ~4 hours at 37oC in a CO2 incubator without shaking. (*Save recovery and plate again if no growth)
- Dilute cells in recovery medium and plate on MRS agar along with the proper concentration of any antibiotics that the plasmid of choice has resistancy (pMSP3535 has resistance to erythromycin).
- Incubate plates for 2 days, then count the colonies present. Allow an additional 3-4 days after initial count to ensure all were noted.
- Cells stored in glycerol (-80 ࠷) after electroporation incubation can be expected to yield similar results to the cells that were plated directly after the incubation.
After preparing the necessary solutions, we followed the Welker protocol with some minor differences. We inoculated bacterial stocks with 10 mL of MRS broth in a CO2 incubator, without shaking, overnight. After this, we subcultured the bacteria in 200 mL of prewarmed MRS broth with 0.9M NaCl from an OD600 of 0.1 until it reached an OD600 of 0.6 (~6 hours). After harvesting the cells by centrifugation and rinsing, we resuspended the cells in 4 mL of cold water and eight 0.5 mL aliquots were divided into 1.5 mL microcentrifuge tubes.
The cells were washed several more times with water and 30% PEG 8000, then stored in a -80°C freezer. These cells should stay viable for up to two years.To enhance the transformation efficiency, 600 μL of prepared cells were suspended 900 μL of cold, sterile, distilled water for 30 min as a pretreatment to electroporation.The cells were pelleted and washed several times with water and 30% PEG 8000. We added 100 ng of plasmid DNA to 100μL of cell suspension as opposed to 200 ng that the protocol recommends; our first plasmid of choice was pMSP3535. This plasmid expresses erythromycin resistance. The cells were electroporated in 2mm cuvettes using the Ec3 settings on the BioRad electroporator which corresponds to 600Ω and 3 kV, differing from the Welker protocol recommendations. After cells were electroporated, they recovered overnight in the appropriate recovery media. They were plated on MRS agar plates with 10μg/mL erythromycin and left to grow for 2 days.
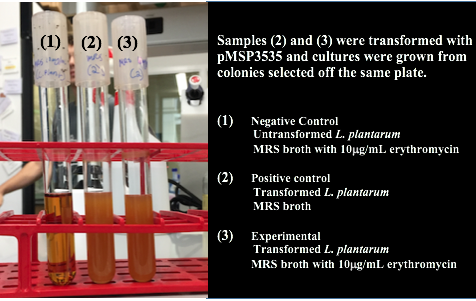
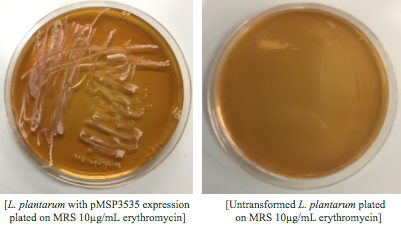
Colonies from each transformation plate were grown up in MRS broth supplemented with 10μg/mL erythromycin. The subsequent day, they were streaked on 10μg/mL erythromycin MRS agar plates to verify resistance.
We aimed to show a visible transformation of Lactobacillus plantarum in addition to the resistance, so we transformed again using a different plasmid. This time we transformed with pBTK501, a plasmid that codes for a resistance to ampicillin and expresses gfp.
References
- Speer, M., & Richard, T. (n.d.). Lactobacillus transformation (Speer 2012). Retrieved May 15, 2017, from https://openwetware.org/wiki/Lactobacillus_transformation_(Speer_2012)
- Landete, J. M., Arqués, J. L., Peirotén, Á, Langa, S., & Medina, M. (2014). An improved method for the electrotransformation of lactic acid bacteria: A comparative survey. Journal of Microbiological Methods, 105, 130-133. doi:10.1016/j.mimet.2014.07.022
- Dennis L. Welker, Joanne E. Hughes, James L. Steele, Jeff R. Broadbent; High efficiency electrotransformation of Lactobacillus casei, FEMS Microbiology Letters, Volume 362, Issue 2, 1 January 2015, Pages 1–6, https://doi.org/10.1093/femsle/fnu033
- de St.Groth, S.F., and Scheidegger, D., Production of monoclonal antibodies: strategy and tactics. J. Immunol. Methods, 35, 1-21 (1980).
- Cox, C. P., & Briggs, M. (1954). Experiments On Growth Media For Lactobacilli. Journal of Applied Bacteriology, 17(1), 18-26. doi:10.1111/j.1365-2672.1954.tb02019.x
- Man, J. C., Rogosa, M., & Sharpe, M. E. (1960). A Medium For The Cultivation Of Lactobacilli. Journal of Applied Bacteriology, 23(1), 130-135. doi:10.1111/j.1365-2672.1960.tb00188.x
- Murphy, M. G., & Condon, S. (1984). Comparison of aerobic and anaerobic growth of Lactobacillus plantarum in a glucose medium. Archives of Microbiology, 138(1), 49-53.