Ianoverman (Talk | contribs) |
Ianoverman (Talk | contribs) |
||
| Line 3: | Line 3: | ||
<h1>Austin_UTexas</h1> | <h1>Austin_UTexas</h1> | ||
<div class = "column full_size"> | <div class = "column full_size"> | ||
| − | </ | + | </p> |
| + | </div> | ||
| − | |||
<h2> Interlab Measurement Study </h2> | <h2> Interlab Measurement Study </h2> | ||
<br> | <br> | ||
<p>The 2017 Interlab Study is the fourth such initiative by the Measurement Committee of iGEM Foundation to establish a hardy measurement procedure for the green fluorescent protein. The goal for the study this year is to test various RBS devices with the intention of finding which combinations yield more stable gene expression and how this might translate to various labs worldwide. Detailed below are the protocols and materials we used during the course of the experiment as provided by the iGEM Foundation. We are proud to contribute to this research endeavor by the iGEM Foundation.</p> | <p>The 2017 Interlab Study is the fourth such initiative by the Measurement Committee of iGEM Foundation to establish a hardy measurement procedure for the green fluorescent protein. The goal for the study this year is to test various RBS devices with the intention of finding which combinations yield more stable gene expression and how this might translate to various labs worldwide. Detailed below are the protocols and materials we used during the course of the experiment as provided by the iGEM Foundation. We are proud to contribute to this research endeavor by the iGEM Foundation.</p> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<br> | <br> | ||
<hr> | <hr> | ||
<br> | <br> | ||
| + | |||
| + | <h3>Protocol: Getting Started</h3> | ||
| + | |||
| + | |||
</html> | </html> | ||
| + | |||
#Transformed Interlab 2017 Part kit devices into <i>E. coli</i> DH5-alpha cells using electroporation. | #Transformed Interlab 2017 Part kit devices into <i>E. coli</i> DH5-alpha cells using electroporation. | ||
#*Plated on LB/CAM and allowed to grow overnight at 37<sup>o</sup>C. | #*Plated on LB/CAM and allowed to grow overnight at 37<sup>o</sup>C. | ||
#*Two colonies from each plate used to inoculate 5 ml of LB/CAM liquid media and grown overnight at 37<sup>o</sup>C and 220 rpm for a total of sixteen culture tubes. | #*Two colonies from each plate used to inoculate 5 ml of LB/CAM liquid media and grown overnight at 37<sup>o</sup>C and 220 rpm for a total of sixteen culture tubes. | ||
#Next dilutions were made in 12 ml of LB/CAM to a target OD<sub>600</sub> of 0.02 and allowed to grow at 37<sup>o</sup>C and 220 rpm in a foil covered incubator with the lights off. | #Next dilutions were made in 12 ml of LB/CAM to a target OD<sub>600</sub> of 0.02 and allowed to grow at 37<sup>o</sup>C and 220 rpm in a foil covered incubator with the lights off. | ||
| − | |||
<html> | <html> | ||
<br> | <br> | ||
| Line 33: | Line 32: | ||
#*Next, 100<span>µ</span>l from these samples were pipetted into the wells of a Costar black with clear-bottom 96 well plate. | #*Next, 100<span>µ</span>l from these samples were pipetted into the wells of a Costar black with clear-bottom 96 well plate. | ||
#*The samples were measured using an Infinite 200 Pro with the following settings: Excitation 485nm with a 9nm bandwidth and an Emission of 530nm with a bandwidth of 20nm. A gain of 48 was also used. | #*The samples were measured using an Infinite 200 Pro with the following settings: Excitation 485nm with a 9nm bandwidth and an Emission of 530nm with a bandwidth of 20nm. A gain of 48 was also used. | ||
| − | |||
| − | |||
<html> | <html> | ||
<br> | <br> | ||
| Line 40: | Line 37: | ||
</html> | </html> | ||
# | # | ||
| + | <html> | ||
| − | |||
| − | |||
<h3>Devices Measured</h3> | <h3>Devices Measured</h3> | ||
</html> | </html> | ||
<p>All parts were located on the pSB1C3 plasmid backbone. The parts used as designated by the provided protocol were:</p> | <p>All parts were located on the pSB1C3 plasmid backbone. The parts used as designated by the provided protocol were:</p> | ||
| − | #Positive Control - BBa_I20270 | + | #Positive Control - <a href="http://parts.igem.org/Part:BBa_I20270">BBa_I20270</a> |
#Negative Control - BBa_R0040 | #Negative Control - BBa_R0040 | ||
#Test Device 1 - BBa_J364000 | #Test Device 1 - BBa_J364000 | ||
| Line 60: | Line 56: | ||
<br> | <br> | ||
<h3>Data</h3> | <h3>Data</h3> | ||
| − | |||
| + | </html> | ||
[[File:T--Austin UTexas--interlabgraph.png|thumb|center|700px|Figure 1. The change of fluorescence over time. 6 different devices were employed in duplicate with a positive and negative control. Samples were collected every 2 hours for a total of 6 hours and afterwards had their fluorescence measured using a plate reader and 96-well plate. Graph produced by Ian Overman.]] | [[File:T--Austin UTexas--interlabgraph.png|thumb|center|700px|Figure 1. The change of fluorescence over time. 6 different devices were employed in duplicate with a positive and negative control. Samples were collected every 2 hours for a total of 6 hours and afterwards had their fluorescence measured using a plate reader and 96-well plate. Graph produced by Ian Overman.]] | ||
| + | <html> | ||
| + | </html> | ||
Revision as of 00:48, 25 October 2017
Austin_UTexas
Interlab Measurement Study
The 2017 Interlab Study is the fourth such initiative by the Measurement Committee of iGEM Foundation to establish a hardy measurement procedure for the green fluorescent protein. The goal for the study this year is to test various RBS devices with the intention of finding which combinations yield more stable gene expression and how this might translate to various labs worldwide. Detailed below are the protocols and materials we used during the course of the experiment as provided by the iGEM Foundation. We are proud to contribute to this research endeavor by the iGEM Foundation.
Protocol: Getting Started
- Transformed Interlab 2017 Part kit devices into E. coli DH5-alpha cells using electroporation.
- Plated on LB/CAM and allowed to grow overnight at 37oC.
- Two colonies from each plate used to inoculate 5 ml of LB/CAM liquid media and grown overnight at 37oC and 220 rpm for a total of sixteen culture tubes.
- Next dilutions were made in 12 ml of LB/CAM to a target OD600 of 0.02 and allowed to grow at 37oC and 220 rpm in a foil covered incubator with the lights off.
Protocol: Plate Reader
- Allow OD600 dilutions to grow for 6 hours at 37oC and 220 rpm
- Take measurements every two hours, starting with hour 0, for a total of four measurement points.
- Measurements were taken by:
- Taking 500µl from each of the sixteen culture tubes and put into microcentrifuge tubes.
- Next, 100µl from these samples were pipetted into the wells of a Costar black with clear-bottom 96 well plate.
- The samples were measured using an Infinite 200 Pro with the following settings: Excitation 485nm with a 9nm bandwidth and an Emission of 530nm with a bandwidth of 20nm. A gain of 48 was also used.
Ludox and Fluorescein Measurements
Devices Measured
All parts were located on the pSB1C3 plasmid backbone. The parts used as designated by the provided protocol were:
- Positive Control - <a href="http://parts.igem.org/Part:BBa_I20270">BBa_I20270</a>
- Negative Control - BBa_R0040
- Test Device 1 - BBa_J364000
- Test Device 2 - BBa_J364001
- Test Device 3 - BBa_J364002
- Test Device 4 - BBa_J364003
- Test Device 5 - BBa_J364004
- Test Device 6 - BBa_J364005
Data
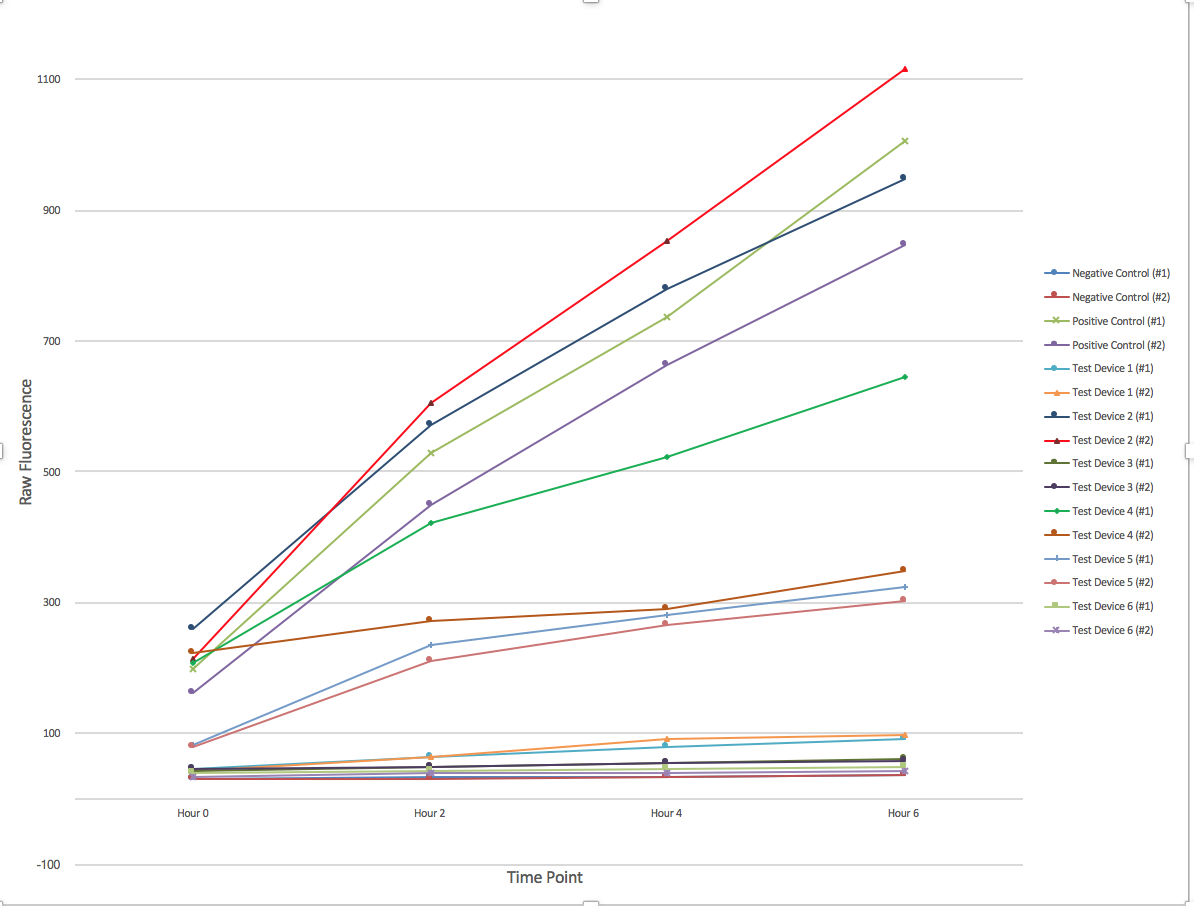
Figure 1. The change of fluorescence over time. 6 different devices were employed in duplicate with a positive and negative control. Samples were collected every 2 hours for a total of 6 hours and afterwards had their fluorescence measured using a plate reader and 96-well plate. Graph produced by Ian Overman.

