| Line 63: | Line 63: | ||
{{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--Microfuildics--cycle.gif|width=1000px|caption=<B>Fig.1 Control the <i>C. elegans</i> by the light.</B> We used the optical fiber to form a light spot and the worm can follow the spot.}} | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--Microfuildics--cycle.gif|width=1000px|caption=<B>Fig.1 Control the <i>C. elegans</i> by the light.</B> We used the optical fiber to form a light spot and the worm can follow the spot.}} | ||
| + | |||
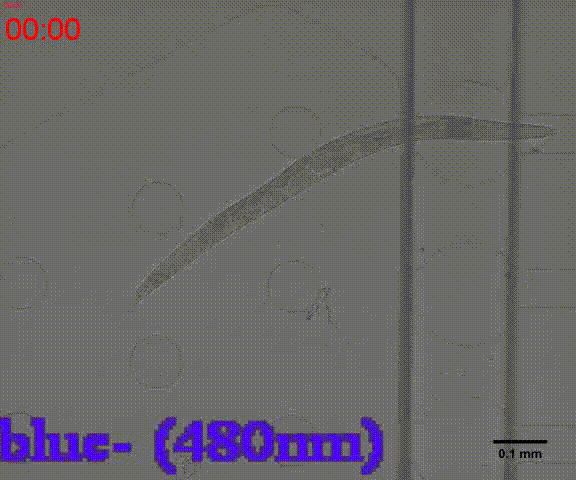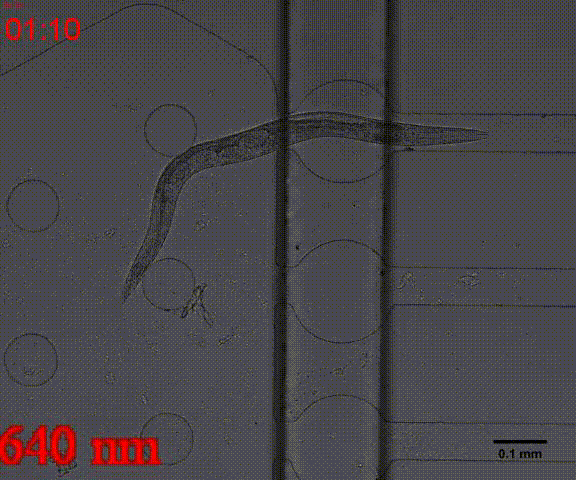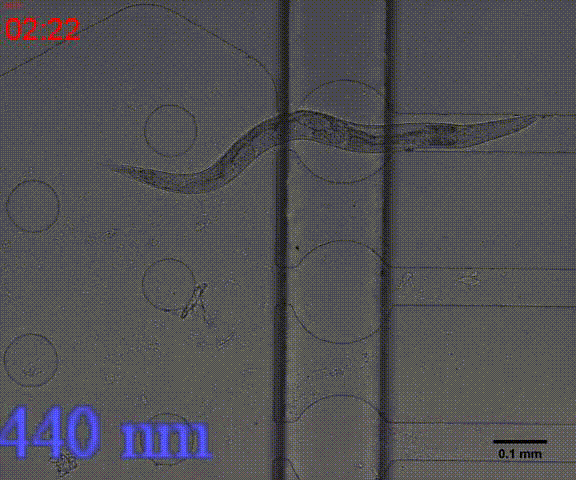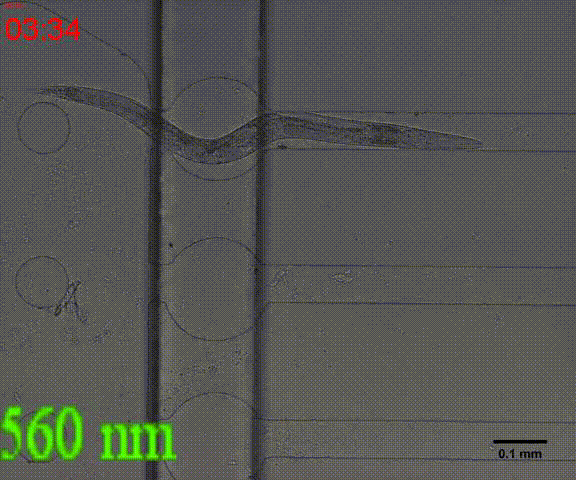
| + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--Microfuildics--wake up.gif|width=600px|caption=<B>Fig.10 The test of the CoChR using light with different wavelengths and intensity. </B>The lights <i>blue-</i> and <i>blue+</i> is from the projector whose wavelength is about 480 and the lights whose wavelengths are 395, 440, 470, 560 and 640 are from the LED of fluorescence microscope (Nikon eclipse Ti).}} | ||
| + | |||
==Alcohol addiction== | ==Alcohol addiction== | ||
Revision as of 13:18, 29 October 2017
Demonstrate
Project
Contents
Overview
So far, our project has been almost completed. The whole project is demonstrated in the following parts:
1.Optogenetic results of spectrum determination
2.Microinjection results
3.Neuron activation
4.Behavior experiment (including single worm and population)
5.Addiction inducing results
Spectrum determination
The channelrhodopsin we chose in our project were evolved from the algae, so if we applied these channel proteins to the C.elegans as the receptors, we need to check its optical parameters before our behavior experiment. The spectrum of Chrimson and CoCHR have been already measured by other scientists, due to their spectrum we need to figure out the another two questions :crosstalk and indicator spectrum.
The first question is that the channelrhodopsin CoCHR will have the wavelength crosstalk with the indicator protein—GEM-GECO , the excitation wavelength of CoCHR is crossed with the emission wavelength of GEM-GECO combined with calcium, which means that we can not activate one protein and receive another protein’s emission together with same wavelengths because of the filter. So we have do the experiment based on the cell to select another suitable excitation wavelength for CoCHR.

The excitation and emission wavelength are the most important parameters for all kinds of optical protein, so if we want know what happened inside the worms’ neuron after light induing, the spectrum of indicators expressed in the C.elegans must be figured out. So we use the sensitive detector to receive the emission wavelength from the C.elegans AWA neuron after activated by diacetyl inducing. Then we analyze the emission data and select the best two wavelength area for the indicator emission wavelength with and without calcium existing. This part will be show in the neuron experiment.
System construction in C.elegans
In order to select the worms stable transacted with the system we have constructed, we design a series selection experiments for verification. These figures showed that the result of the selection process using the fluorescence marker selection ,the rescue selection and the negative poison selection. We then do the mapping for the genome of the worms to check the insertion of the system. Finally we use confocal microscope to check the location marker to test the expression of the whole system.

Activation of C.elegans head neurons
Since the whole system has expressed in the C.elegans, we need to check the function of each part in our project. So we have done many experiments on the head neuron of the worms, we first check the function of the GEM-GECO.
These figures showed the position and the reaction of AWA neuron which expressed GEM-GECO after diacetyl inducing.
Microfluidics
In order to study locomotion of C. elegans populations, we design the Gaussian Chip, a pillar-filled area, where the pillars are designed such that they allow crawling-like behaviors even though worms are immersed in liquid environment.


Response to light (Individual)
To verify the response to specific wavelength light inducing, we pick out single worm to observe the behavior change.
Alcohol addiction