Design
Project
The aim of our project is to construct a learning platform in C.elegans. This platform should be able to provide us the ability to observe and control the neurons’ activity in order to study the process of behavior formation and finally figure out the neural-network level roots of learning ability.
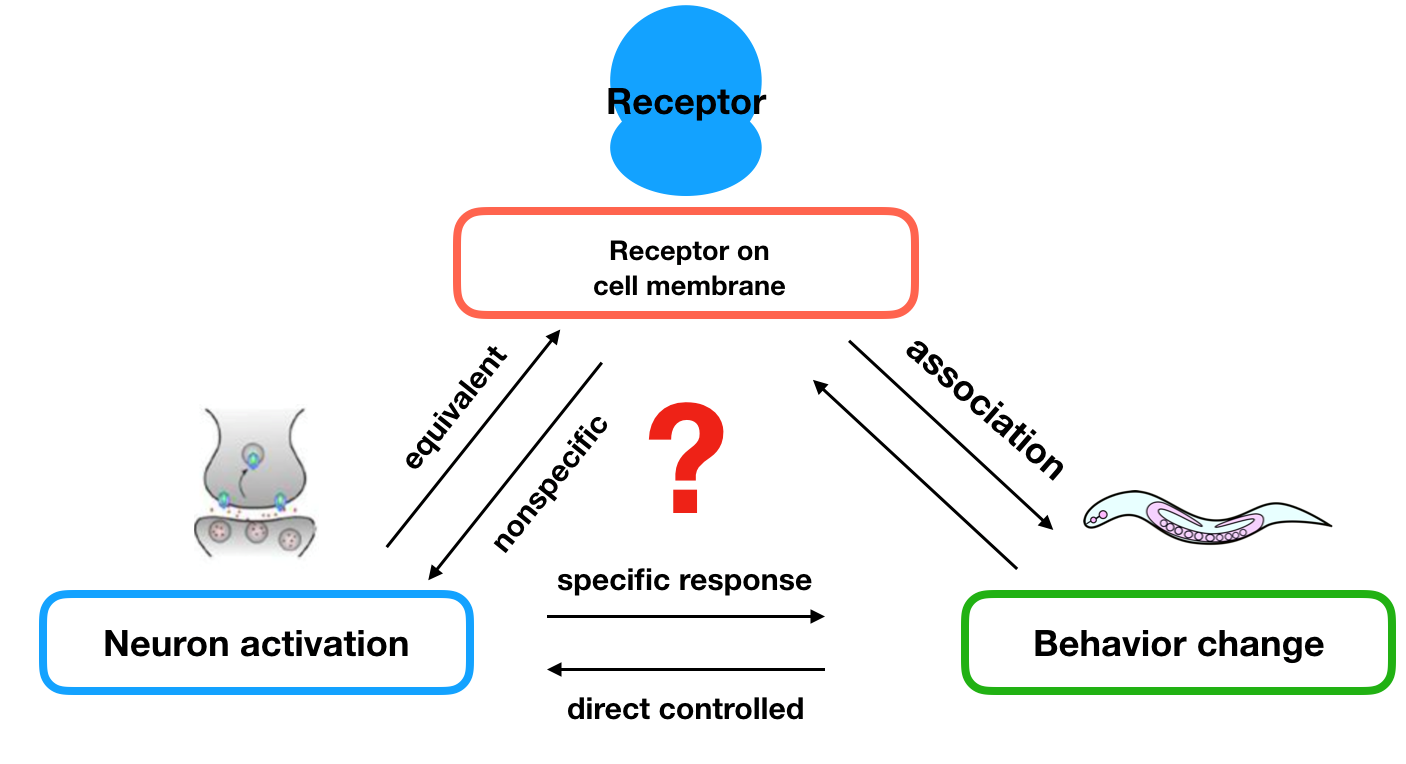
To achieve this goal, we first need to link neuron activation with behavior change.
The neuroscientific research[1] of C.elegans revealed that a pair of olfactory neurons of C. elegans, namely AWA and AWB, play the important roles on determining the preference to different odorants. For example, the attractive odorant diacetyl is detected by the receptor protein Odr-10 in AWA neuron and the repulsive odorants is detected by the receptor protein Str-1 in AWB neuron. However, transgenic worms that express Odr-10 in AWB rather than AWA will avoid diacetyl. That gave us the inspiration that expression of other exogenous receptors on these two neurons can also lead to their preference to certain substances.
To figure out how the olfactory neurons affect behavioral change, we applied two hypotheses:
- the neurons are equivalent when equipped different types of receptors;
- if the stimulations are not sensed by other receptors, we can control the preference of nematodes towards different stimulations by changing different receptors on these two neurons.
These hypothesis were supported by the experiments done by other scientists, the experiment showed that we can transfer an exogenous receptor onto the neuron using a promoter that express in one of the pair of neurons , and the transferred receptor will lead to avoidance (AWB) or preference (AWA). That is to say, if the stimulations are not sensed by other receptors, we can control the preference of them towards different stimulations by changing unique receptors on these two neurons. Because of these, we can apply our receptor to these two neurons to achieve our aim.
Next, we need to design the gene circuit to achieve our goal.
Wetware Engineering
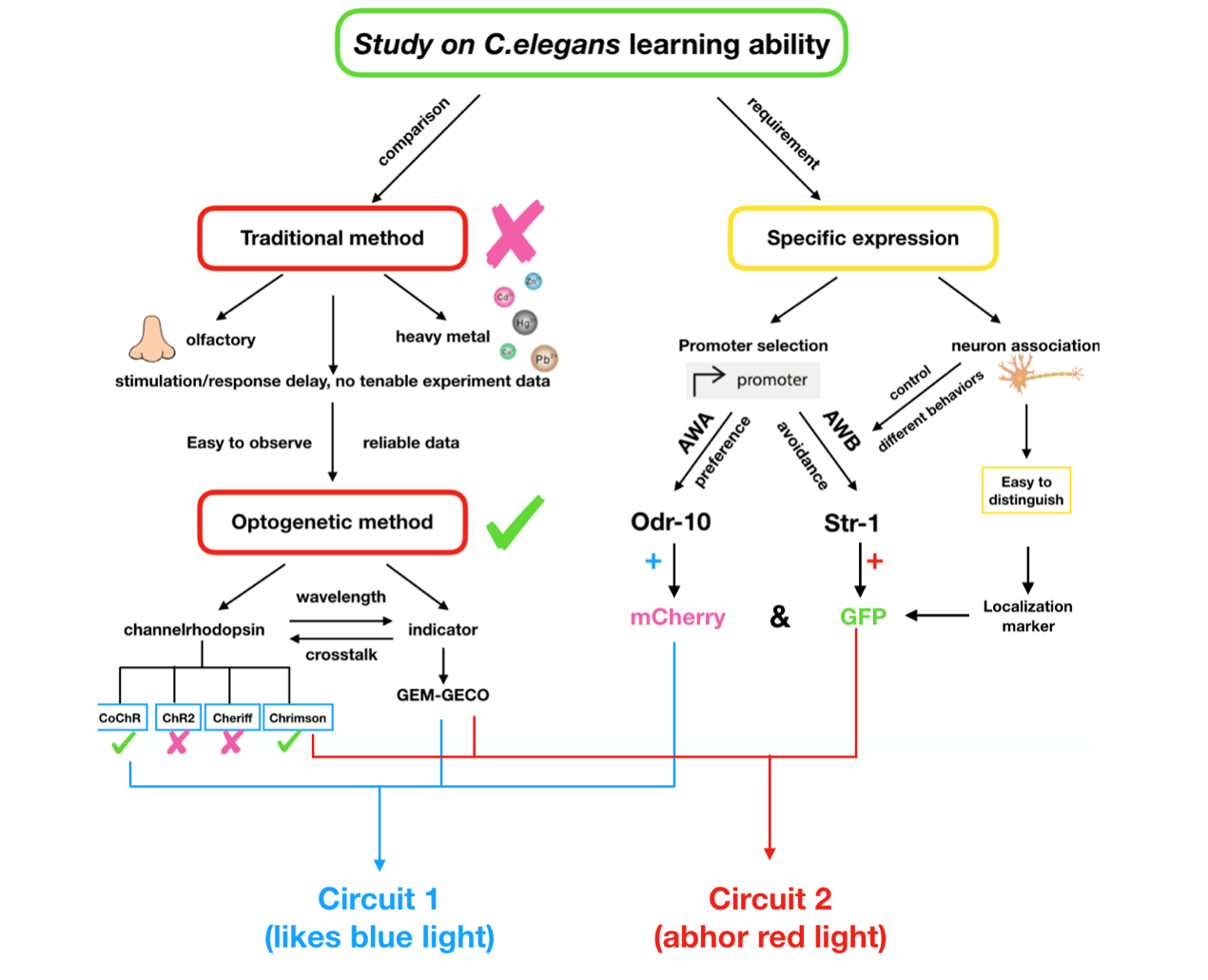
We need to construct a system inside the worms which can make them show specific response to the inducing signal, and form the learning behavior after training. However, former researches of learning ability based on C.elegans were more focused on chemical signals like olfactory inducing, heavy metal stimulus. These methods can surely demonstrate the existence of the learning behaviors but seem not tenable as reliable experimental data, since the residue of the stimuli is difficult to erase. Thus we choose optogenetic method to realize this goal.
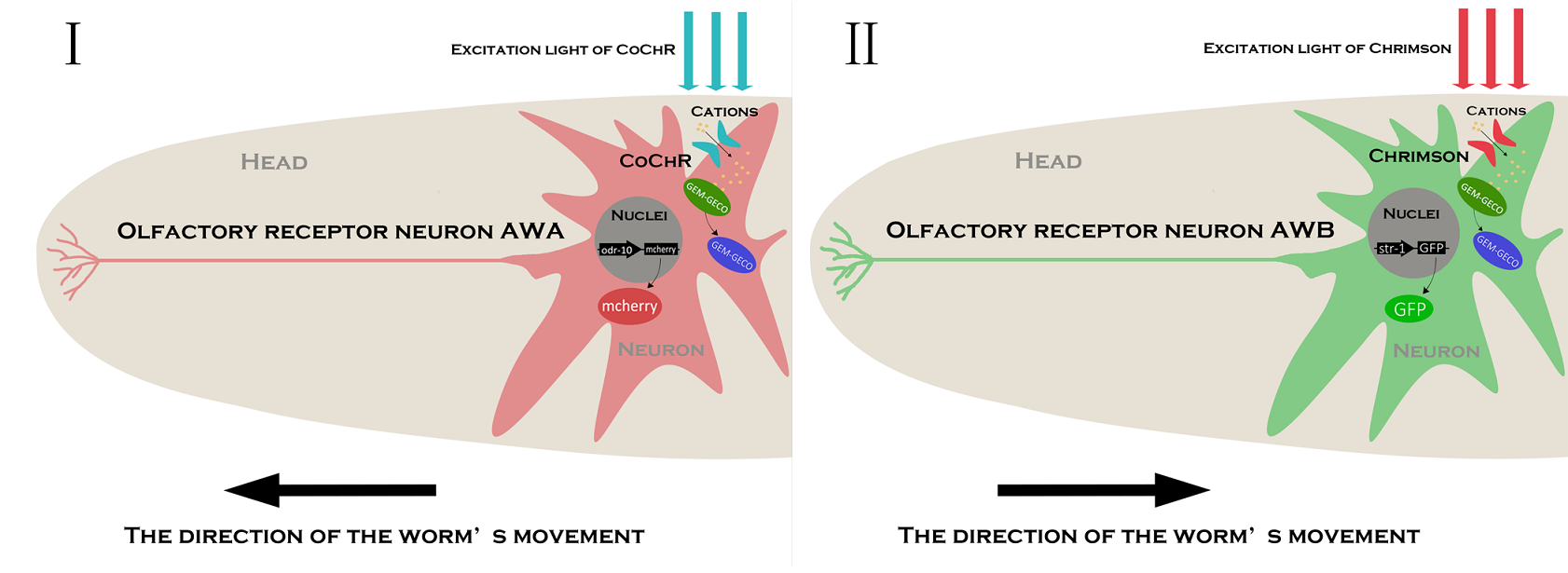
In order to express the functional components in specific neurons (Pairs of olfactory neurons: AWA and AWB in our project), we use the promoter of Odr-10 (specifically expressed in AWA) and Str-1 (specifically expressed in AWB) respectively in AWA and AWB. The reason why the promoter from cell-specific protein enables expression of target components is demonstrated by the published paper[2]. Thus, if the channelrhodopsin(Channelrhodopsins are a subfamily of retinylidene proteins that function as light-gated ion channels.) expresses in specific cells of organisms, with a specific wavelength of light stimulation, we are capable of activating or suppressing the specific ion channel to manipulate the condition of the target cell.
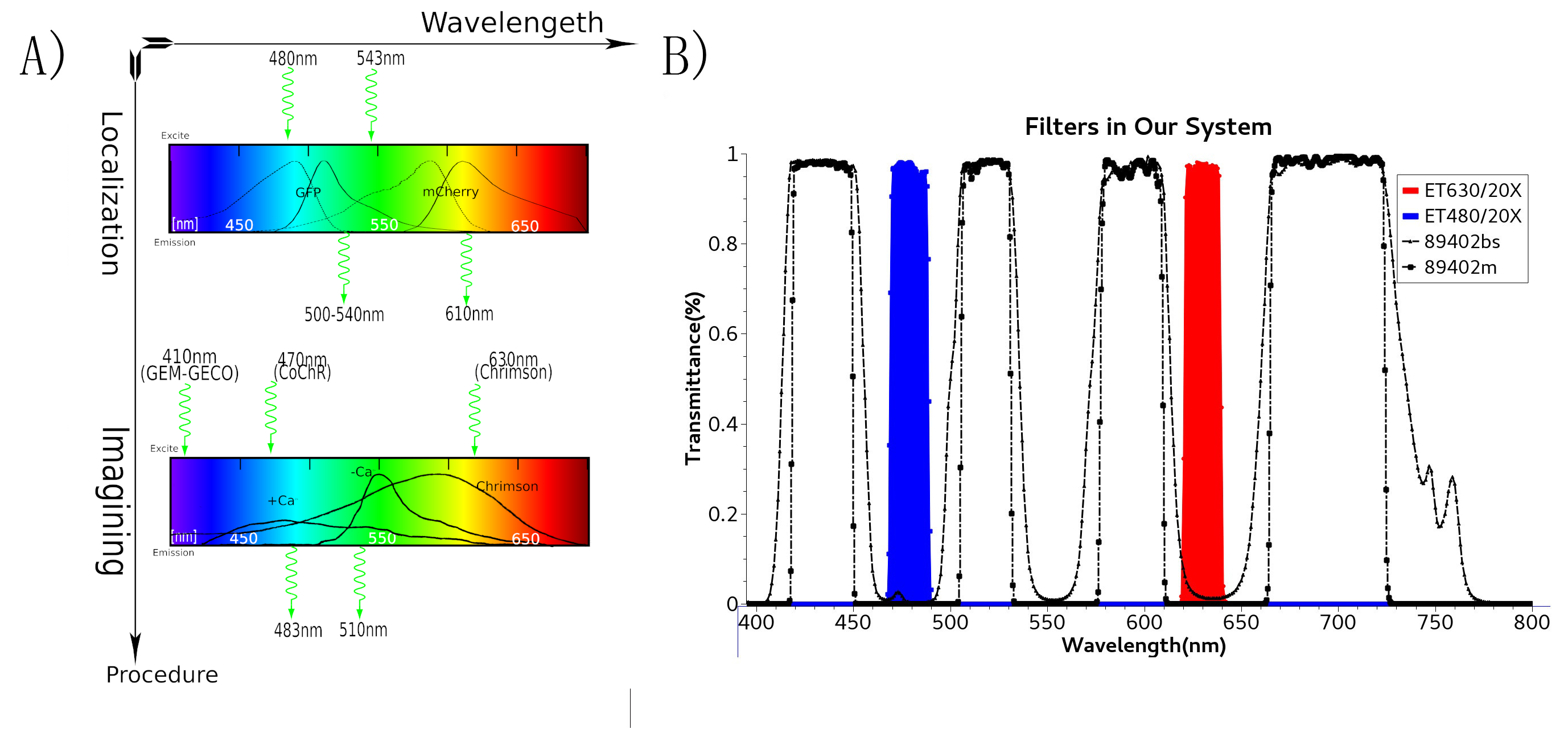
To study the nematodes’ behavior with optogenetic method, we have to decouple the crosstalk of the light sensing elements. In our final design, we chose a pair of channelrhodopsins, the red-light sensitive Chrimson and the blue-light sensitive CoChR. The channelrhodopsins intensely used in previous research, ChR2 and C1V1, have a large overlap on the excitation spectrum which indicates the high risk of cross-activation. CoChR is chosen because it is five times more sensitive to blue light than the commonly used ChR2, so low-intensity blue light can be used without triggering cross-activation of Chrimson. These two channelrhodopsins are sensitive to light of certain wavelength (blue light stimuli correspond to 400 to 520nm band and the red light stimuli correspond to 600 to 725nm band).
In practice, by making a plasmid containing original receptor’s promoter, channelrhodopsins, Ca2+ indicators and fluorescence proteins, the light sensitive receptors and other proteins can be expressed in C.elegans. Artificial introns are inserted in the genetic sequence of the plasmid, to enhance their expression in the nematodes. For the sake of ensuring efficient concomitant and equivalent expression of more than two polypeptides from a single promoter, viral 2A peptides are used, which trigger a “ribosomal-skip” or “STOP&GO” mechanism during translation, to express multiple proteins from a single vector in C.elegans.
Hardware Engineering
In the whole circuit in AWA neuron, blue light of 470 nm wave length activate CoChR channel. GEM-GECO, as the calcium indicator, is activated at 395 nm, and shift its emission wavelength from 542 nm to 483 nm. In the whole circuit in AWB neuron, red light of 630 nm wave length activate Chrimson channel. Also, we will capture the wavelength range of GEM-GECO after neural activation.
Thus, to precisely control the light stimuli and receive all the output signals, a customized optical device is needed. Beside, to study the collective behavior of worms with high throughput, an accurate and user-friendly is required.
Compared with other model organisms like Mus musculus, which needs approximately two to three months to mature, C.elegans possesses shorter life span. A self-fertilizing hermaphrodite can maintain its genetic information and live for two to three weeks[8]. Meanwhile, worms can also propagate sexually to get genetic recombination offsprings.
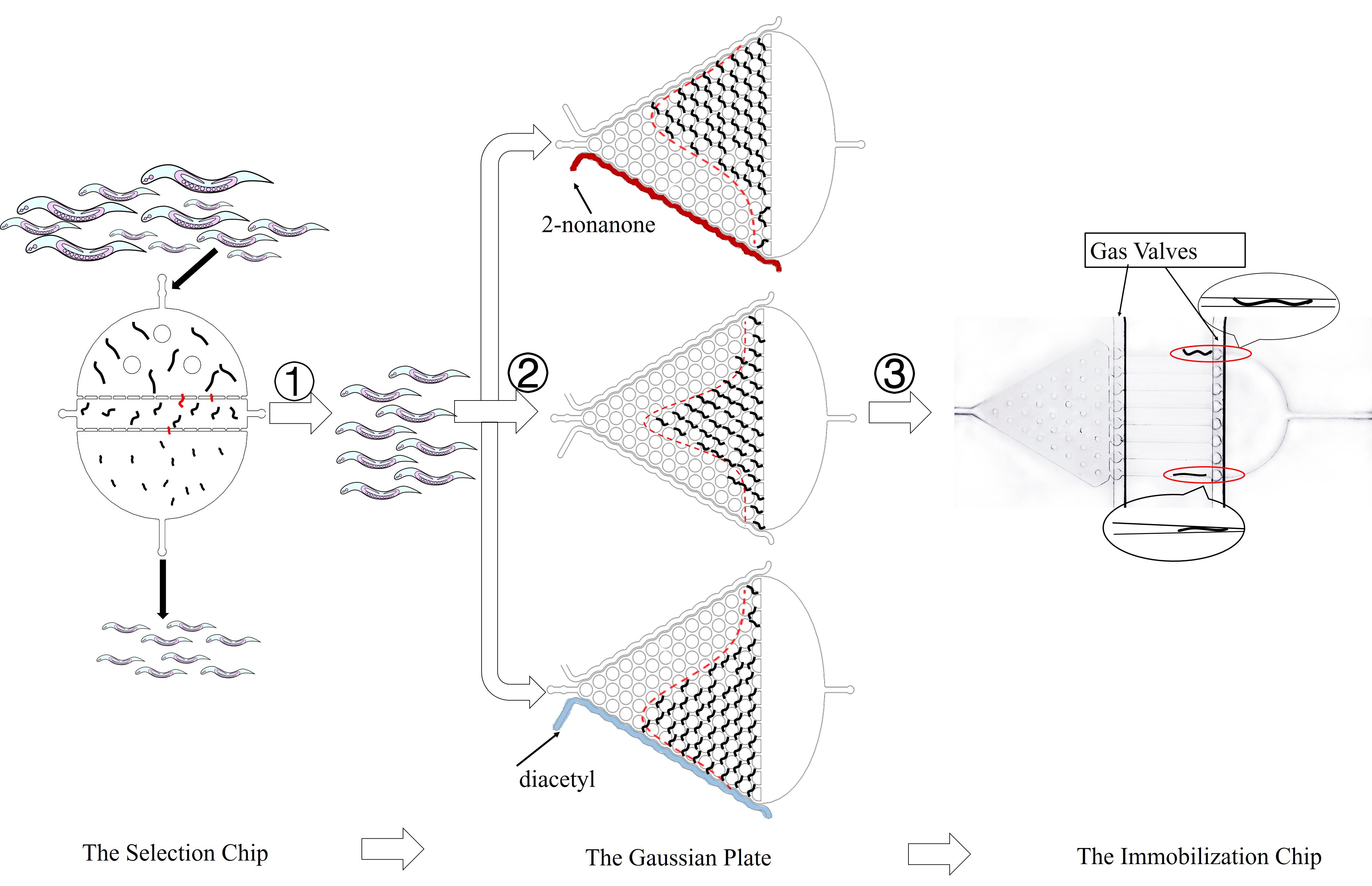
In our project, we choose the olfactory neurons ( AWA &AWB) to accomplish our design. The attractive odorant diacetyl is normally sensed by the AWA olfactory neurons. The repulsive odorant 2-nonanone is detected by the AWB olfactory neurons. With the expression of CoChR and Chrimson(photosensitive proteins) in these neurons, worms can develop attractive and repulsive responses to blue light and red light, so we can use lights to induce their learning behaviors.
There are several manipulandums in the box, which can automatically detect the occurrence of a behavioral response or action. Typical operands for primates and rats are response levers; if the animal presses the lever, the opposite end moves and closes a switch that is monitored by a computer or other programmed devices. When the lever is pressed, food, water, or some other type of reinforcements (a consequence that will strengthen an organism's future behavior whenever that behavior is preceded by a specific antecedent stimulus.) might be dispensed. In some instances, the floor of the chamber may be electrified. The rats learn to press the lever through several training periods because of awards or punishments. [11]
In our project, we train the transgenic C. elegans and control their behaviors by lights. Expression of two channelrhodopsins in the olfactory receptor neuron pair provides worms with the preference or aversion to specific wavelengths, and the corresponding lights are employed to reinforce their addictive or abstemious attitude to alcohol. Those reflects or behaviors are all operant behaviors and operant reflects which can be helpful for a more deep studies concerning nerves and behaviors.
Optogenetics can stimulate and monitor the activities of individual neurons within freely-moving animals and precisely measure their response in real-time. In our project, it makes it possible to train worms, observe their behaviors and quantify the neuronal signals. In traditional training experiments, the simulation (input) is always has an inevitable residue, from hours to days. Besides, the readouts(output) are not in the same pace with the optical control. However, by using our optical device(see hardware-link), these issues can be solved greatly.
Despite the fact that worms are confined in chips and pushed by the fluid, the channels are specially designed to effectively simulate their normal movements. PDMS, the material of the chips, is transparent and has no influence on the quantification of light signals in optogenetics experiment. Gas molecules, diacetyl and 2-nonanone, can diffuse in PDMS so that worms can sense the odor of such molecule. As a result, we can observe their preference to these odors.
In our design, we use a group of optogenetic proteins and optical devices. Channelrhodopsins protein CoChR and Chrimson are coupled with dual-Color fluorescence protein GEM-GECO and expressed in the pair of neurons AWA and AWB. This combination is used to lower the cross-talk and to get clearer image during experiments. Excited by blue light and red light separately, CoChR or Chrimson can induce the change of calcium ion concentrations inside the nerve cells. Then it can cause the blue-shift of fluorescences of GEM-GECO. To enhance preciseness, we modified the projector and design our own software of microscope. New filters are installed in the device so that we can simultaneously control the intensity and period of light when stimulating the individual C. elegans during manipulation.
That is why our project is so important.
It employs the optogenetics and neuroimaging to record the change in each neuron during the formation of new ability, so we can know what happened exactly inside the brain. Moreover, the application of microfluidics in our platform allows us to combine the process among ability-training and neuron-activating, and makes the research becoming easier to carry out.
In the future, our platform can also be applied in different fields, such as the simulation of the Brain-Computer Interface (BCI). The BCI can help the paralysis patients obtain the ability to move and operate things. But the signals from the human brain is too complex and difficult to deal with. If we can decipher the neuronal signals during learning progress when the patient’s mind interacting with the computer, it is possible to simplify the network and build more connections between them.
We are now using our platform to study the alcoholism by trainning worms to be addicted with alcohol and then study the neuron networks during the formation of this behavior. Hope one day we can figure out the logic networks of alcoholism and help to treat more serious social problem.
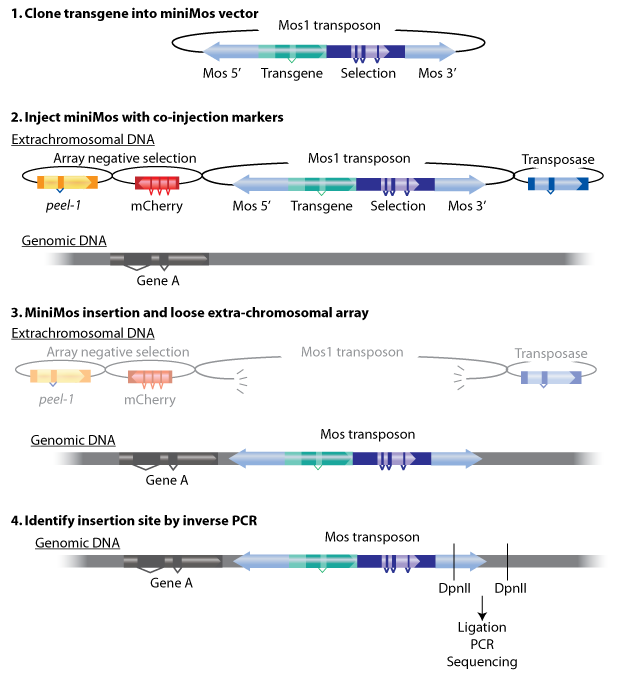
The miniMos system contains 4 vectors——transposon with target gene, transposase, mcherry and peer-1 markers. Transposon needs transposase to cut and paste in the genome. mcherry and peer-1 are two negative selecter that can help to select the target strains.
The method has some advantages:
1)The insertion frequency and fidelity is high.
2)The exact insertion site can be determined.
3)Large transgenes can be inserted.
4)The miniMos element is active in C. elegans.
5)Transgenes are expressed in the germline at high frequency.
In our program, we aim to change the physical behaviour of C. elegans by using the two specific optogenetic traits. Through microinjection and selection, we are able to get two strains with two phenotypes of the preference to blue light and the aversion to red light, and the next step is to obtain the single worm with the combination of these 2 different traits.
To achive our objectives, we choose a mating method, because it is less time-consuming and easier to operate than microinjecting the other array to the existed strain.
In general, mating is the pairing of either opposite-sex or hermaphroditic organisms. However, for C. elegans, mating can hybridize two different traits on the next generation by the paring of the hermaphrodite and the male. Besides, one of the greatest advantages of C. elegans is that it can either autocopulation or hybridization, which means that after hybridization, the hermaphrodite can self-fertilize and guarantee to produce a large number of offsprings with identical genetic characteristics.
Here is the mating procedures:
1.Heat shock to get males(30℃,8h).
2.Parents mating to get the first filial hybrid generation(female : male = 3 : 4).
3.Single out each hermaphrodite in a small plate.
4.Test the fluorescence of F1.
5.Single out F1 and self-fertilizing to get F2.
6.Test the fluorescence of F2.
References
- ↑ Emily R. et al. (1997). Reprogramming Chemotaxis Responses: Sensory Neurons Define Olfactory Preferences in C. elegans, Cell, Vol. 91, 161–169.
- ↑ Emily R. et al. (1997). Reprogramming Chemotaxis Responses: Sensory Neurons Define Olfactory Preferences in C. elegans, Cell, Vol. 91, 161–169.
- ↑ Caenorhabditis elegans(WIKIPEDIA). Retrieved September 25, 2017 https://en.wikipedia.org/wiki/Caenorhabditis_elegans
- ↑ Brenner, S., Draft, A. N. C., Chklovskii, D., System, F. F. N., Brain, T. M., & Seung, S., et al. . The connectome debate: is mapping the mind of a worm worth it?. Scientific American
- ↑ Caenorhabditis elegans(WIKIPEDIA). Retrieved September 25, 2017 https://en.wikipedia.org/wiki/Caenorhabditis_elegans
- ↑ Chen, Y., Scarcelli, V., & Legouis, R. (2017). Approaches for studying autophagy in caenorhabditis elegans. Cells 6(3)
- ↑ Caenorhabditis elegans(WIKIPEDIA). Retrieved September 25, 2017 https://en.wikipedia.org/wiki/Caenorhabditis_elegans
- ↑ Uno, M., & Nishida, E. (2016). Lifespan-regulating genes in C. elegans. , 2, 16010.
- ↑ R.Carlson, Neil (2009). Psychology-the science of behavior. U.S: Pearson Education Canada; 4th edition. p. 207. ISBN 978-0-205-64524-4.
- ↑ [Krebs, John R.(1983). "Animal behaviour: From Skinner box to the field". Nature. 304 (5922): 117. Bibcode:1983Natur.304..117K. PMID 6866102. doi:10.1038/304117a0
- ↑ Brembs, Björn. "Operant conditioning in invertebrates". Current Opinion in Neurobiology. 13 (6): 710–717. doi:10.1016/j.conb.2003.10.002
- ↑ Editorial, N. (2011). Method of the year 2010. Nature Methods.
- ↑ Chung, K., et al. (2008). ”Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans.” Nature Methods 5(7): 637-643.