| Line 92: | Line 92: | ||
pA and pB are the partial vapor pressure of component A and component B in the mixture, they follow the Raoult’s law: | pA and pB are the partial vapor pressure of component A and component B in the mixture, they follow the Raoult’s law: | ||
| − | {{SUSTech_Shenzhen/bmath|equ=<nowiki> p_A= x_A\cdot p^*_A </nowiki>}} | + | {{SUSTech_Shenzhen/bmath|equ=<nowiki> p_A= x_A\cdot p^*_A\qquad p_B= x_B\cdot p^*_B</nowiki>}} |
| − | + | ||
| + | where p^*_A is the vapour pressure of pure A and p^*_B is that of pure B. The total vapor pressure p of the mixture is therefore: | ||
| + | {{SUSTech_Shenzhen/bmath|equ=<nowiki> p= p_A+p_B =x_Ap^*_A + x_Bp^*_B= p^*_B+(p^*_B-p^*_A) </nowiki>}} | ||
Revision as of 13:49, 1 November 2017
Chemical Diffusion
Model
An experiment is designed to demonstrate that the insertion does not damage the olfactory receptor neuron pairs by testing worms’ response to diacetyl and 2-nonanone. We assume that if the neuron pair is unaffected, the modified worms will respond to the chemicals as the wild types do.
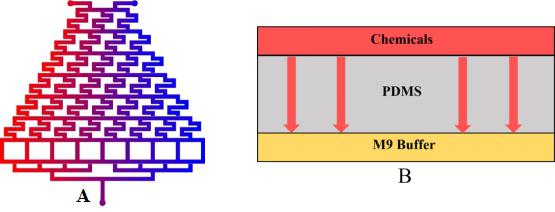
To warrant the success of the experiment, we have 2 plans to form a concentration gradient in the microfluidic chip.
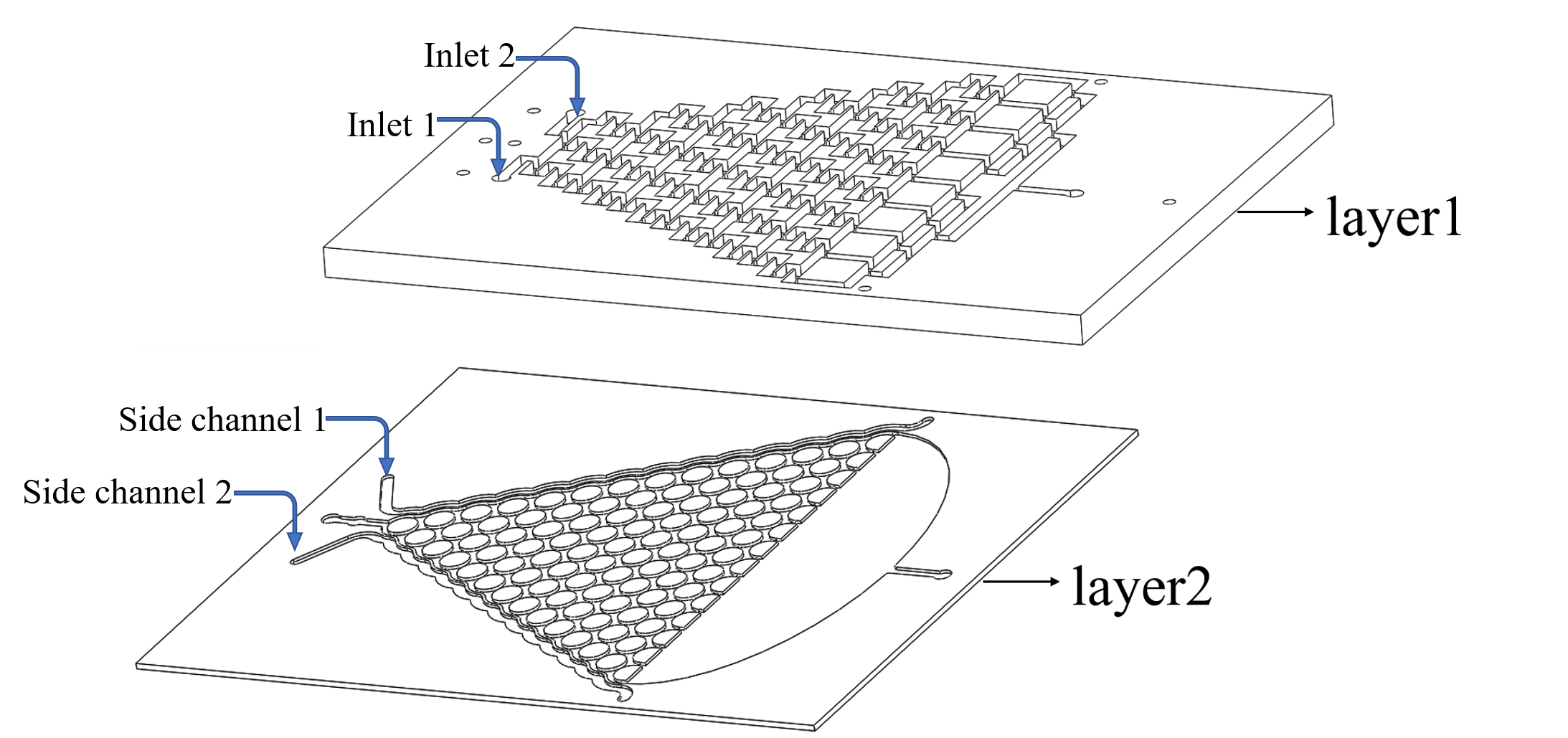
Plan A: Use layer1 to mix the chemicals from the inlets.
Plan B: Input the liquid to the side channel in layer2. (Fig.1)
In this model, we only concern the situation in Plan A because ideally this method can lead to more precise and uniform concentration than Plan B dose.
Considering the time consumption of the diffusion process, we need to determine the Delaying Time (the interval between the time of adding chemicals and the time we start to count the worms) in our experiment. The Delaying Time is related to two processes:
• We inject the chemicals into the first layer to form a concentration gradient (Fig.2).
• Gas molecules diffuse downward across the PDMS layer and reach the second layer.
After that the worms in the Layer2 can respond to the gradient chemical (diacetyl or 2-nonanone). We are able to determine whether their behavior is close to the wild types’See another model.
Physical Process
1.The Form of the Concentration Gradient
We need to know the time for forming the uniform concentration gradient. The characteristic diffusion time tc can be calculated by the Einstein and Brownian movement equation:
t=\frac{w^{2}}{2D}
Here, w is the diffusive distance, which is equal to half of the width of the branch channel (In our work, the width of any serpentine branch channel is 0.15 mm.);
D is the diffusion coefficient of the molecule.
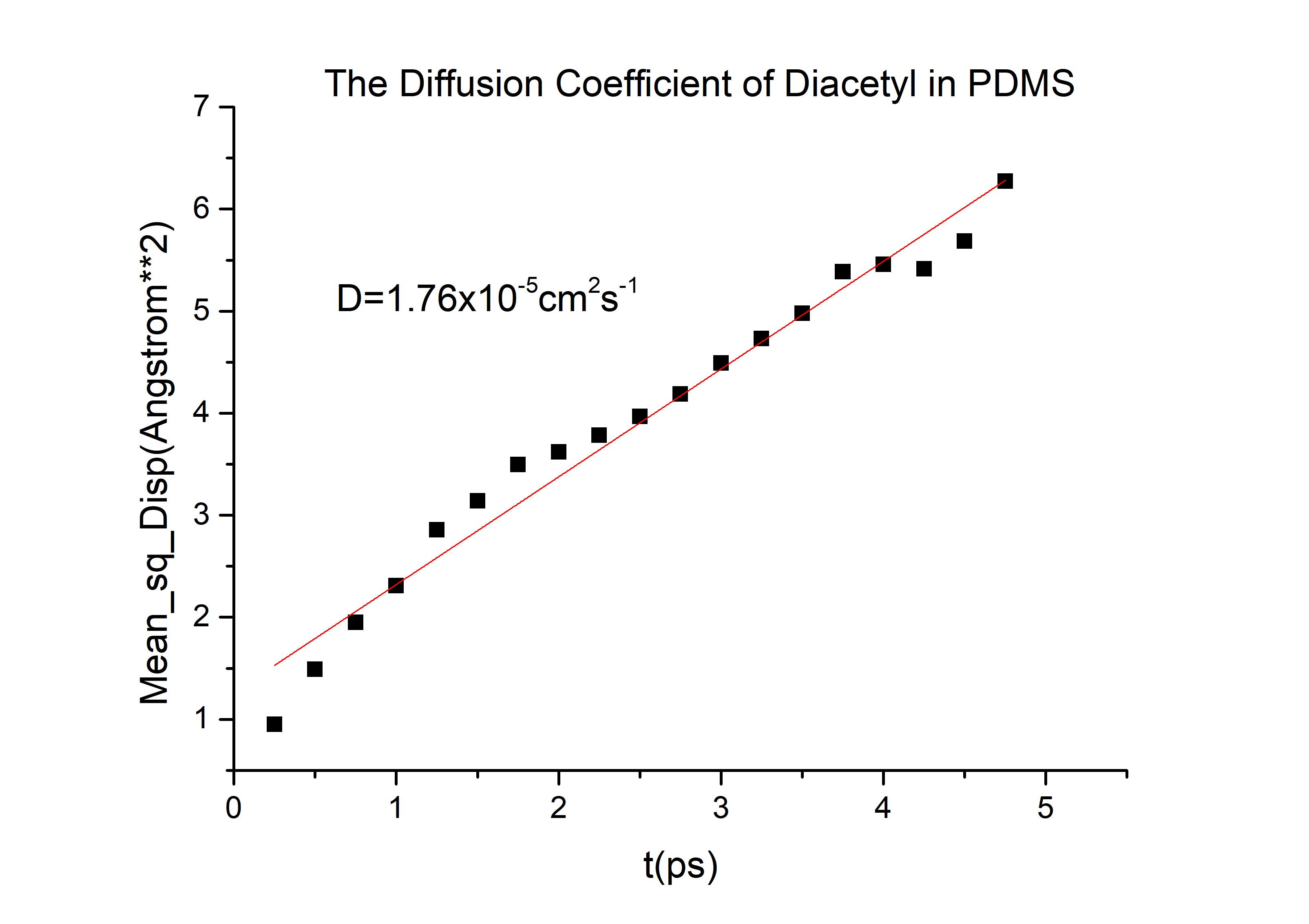
The D value of the diacetyl is 8.5*10-6cm2s-1
Based on the above formulas and parameters, we can get our t_c=3.3s. It means that it costs 3.3s to form the expected gradient in layer1.
2.The Diffusion Time for Chemicals in PDMS
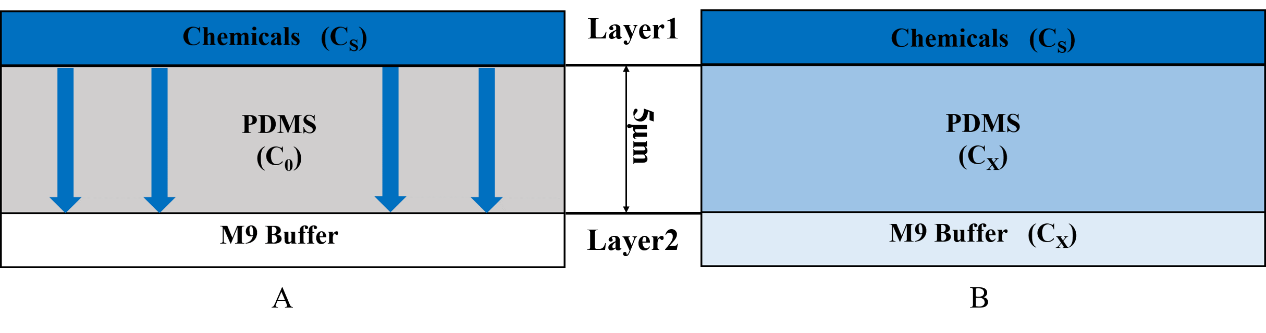
The process that gas molecules diffuse downward across the PDMS layer is considered. The second layer is full of the continual M9 buffer flow, so the chemicals will not be accumulated in this layer. We can assume that the concentration of layer2 is always equal to the concentration at the bottom of the PDMS.
A simplified diffusion model is built to describe the physical process:
For nonsteady-state diffusion, we use Fick’s second law to describe the process
\frac{C_x-C_0}{C_s-C_0}=1-erf(\frac{x}{2 \sqrt{Dt}})
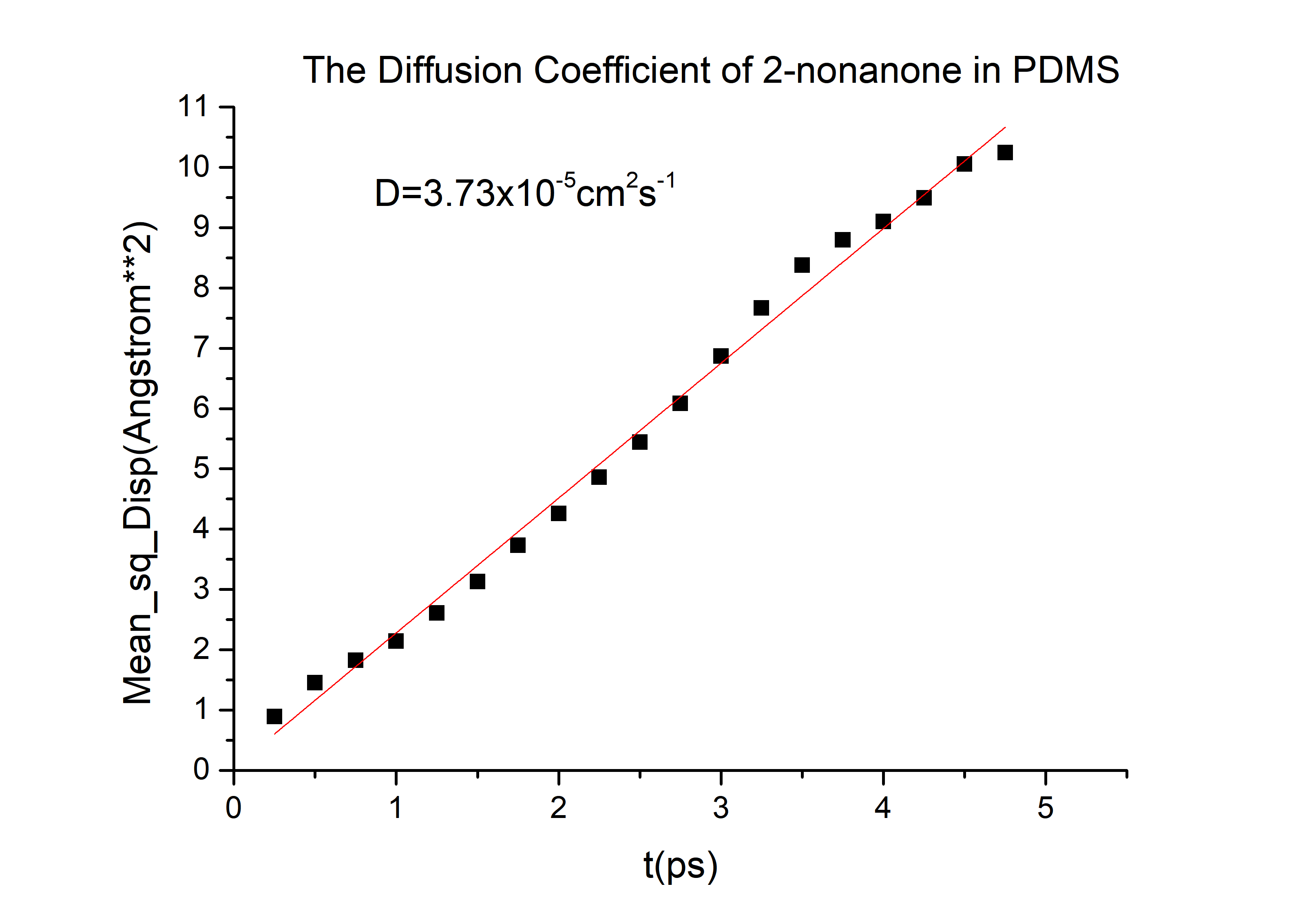
D(2-nonanone) = 6.78*10-6cm2/s in PDMS.
D(diacetyl) = 6.78*10-6cm2/s in PDMS.
Calculation details are shown in The Calculation of Diffusion Coefficient D
Concentration C is related to the position (distance x within PDMS) and the time variable t.
For t=0, C=C0 at 0≤x≤∞;
For t>0, C=Cs (the constant surface concentration) at x=0;
C=C0 at x=∞. And then there is a general solution: Where Cx represents the concentration at distance x after time t.
\frac{x}{2 \sqrt{Dt}}=constant, so if this constant can be calculated, the equation of distance x and time t can also be gotten.
In our model, C0=0, so the equation is changed to \frac{C_x}{C_s}=1-erf(\frac{x}{2 \sqrt{Dt}}) Cx represents the concentration at distance x that C. elegans can feel.
Cs is the concentration set by our own and it is a constant. x is 5mm (Fig.4).
We need to know the fractions of the chemicals in the gas because only the gas molecular can across the PDMS.
For ideal mixture of the two pure liquids, the compositions of the liquid and vapor follows from Dalton’s law that the mole fractions in the gas, yA (the mole fraction of diacetyl) and yB (the mole fraction of water) [2], are
y_A=\frac{p_A}{p}\qquad y_B=\frac{p_B}{p}
pA and pB are the partial vapor pressure of component A and component B in the mixture, they follow the Raoult’s law:
p_A= x_A\cdot p^*_A\qquad p_B= x_B\cdot p^*_B
where p^*_A is the vapour pressure of pure A and p^*_B is that of pure B. The total vapor pressure p of the mixture is therefore:
p= p_A+p_B =x_Ap^*_A + x_Bp^*_B= p^*_B+(p^*_B-p^*_A)
The constant of proportionality D is called the diffusion coefficient, which is expressed in square meters per second. Concentration C is plotted versus position (or distance) within the solid x and the time t.
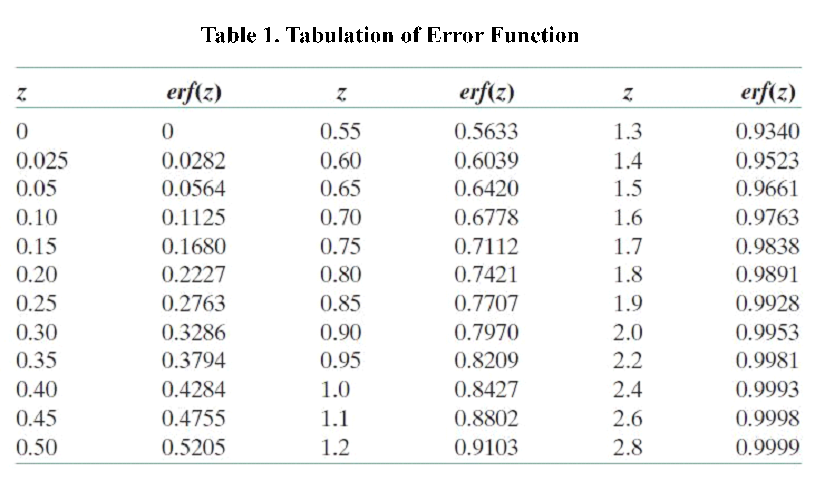
According to the equation we can get that the erf (\frac{x}{2 \sqrt{Dt}})=0.9965 and then referred to the Table 1.1 we can get that the \frac{x}{2 \sqrt{Dt}}=2.2, and D is the diffusion coefficient for 2-nonanone or diacetyl in PDMS. We need to calculate the D to get the time t according to the equation. We used the force-field method to get the D of those two chemicals (the concrete operational process is shown in 1.3).
D(2-nonanone) = 6.78*10-6cm2/s
D(diacetyl) = 6.78*10-6cm2/s
Finally, we can get the time t = 12 mins, on the other words, we cannot inject the C. elegans until the chemicals are injecting for 12 mins in layer 1(for diacetyl).
The Calculation of Diffusion Coefficient D
The diffusivity of a gas in an organic solvent, polymer, or zeolite can be calculated by simulating the molecular dynamics process and calculating the mean square displacement of the gas in the material. This allows us to calculate the self-diffusivity coefficient of the gas and gives an insight into the overall diffusivity. As we are performing a molecular dynamics calculation, we can analyze the effect of temperature, pressure, density, and penetrant size and structure on diffusion. We used force-field method to calculate the D in PDMS by Material Studio® (MS) a software for material calculation.[1][2]
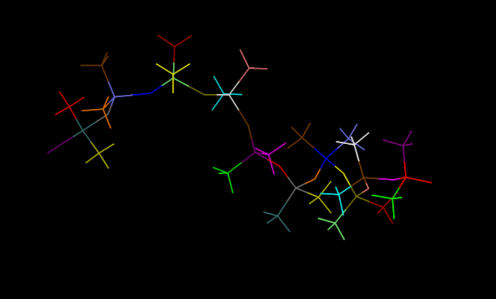
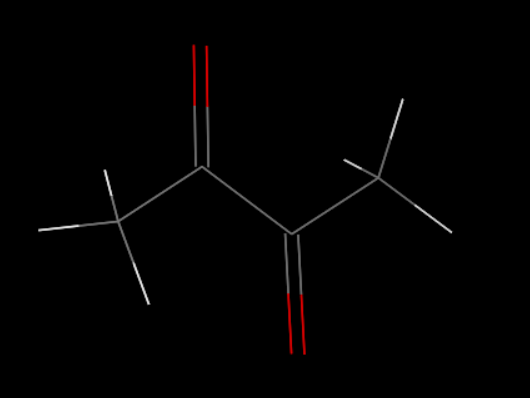
Set up the initial structures
First, we set up the initial structure of the PDMS and the chemicals (Fig.5 and Fig.6).
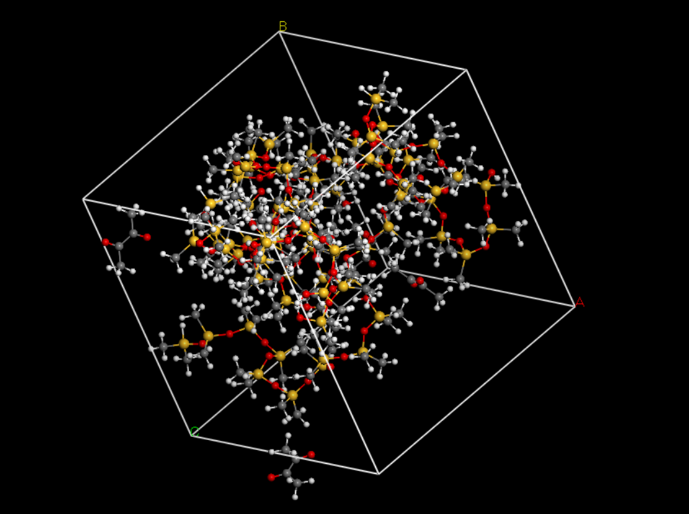
Build an amorphous cell
Then, we put the two molecules in to an amorphous cell (Fig.7).
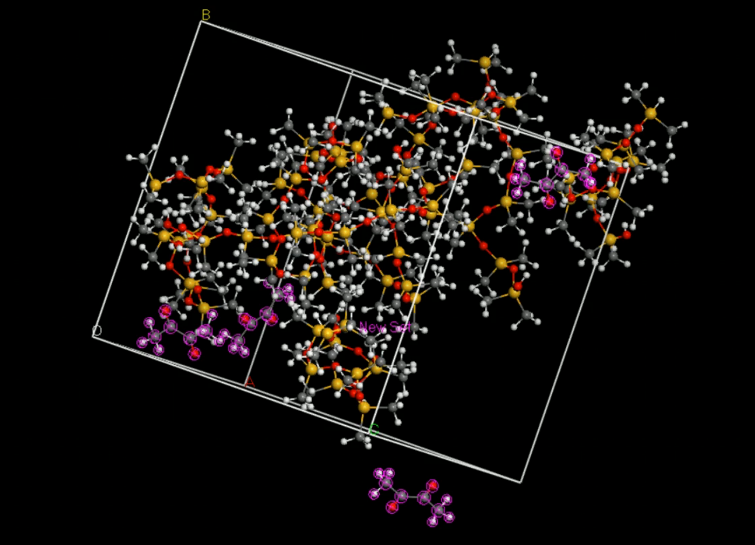
Relax the cell
When we generate an amorphous cell, the molecules may not be equally distributed throughout the cell, creating areas of vacuum. To correct this, we must perform a short energy minimization to optimize the cell. After the minimization, we should run a short molecular dynamics simulation to equilibrate the cell.
Run and analyze molecular dynamics
Export data and calculate the diffusivity
Then we can got the D of 2-nonanone and diacetyl:
D(2-nonanone) = 6.78*10-6cm2/s
D(diacetyl) = 6.78*10-6cm2/s
References
- ↑ S. G. Charati† and, & Stern, S. A. (1998). Diffusion of gases in silicone polymers: molecular dynamics simulations. Macromolecules, 31(16), 5529-5535.
- ↑ Hofmann, D., Fritz, L., Ulbrich, J., Schepers, C., & Böhning, M. (2000). Detailed‐atomistic molecular modeling of small molecule diffusion and solution processes in polymeric membrane materials. Macromolecular Theory & Simulations, 9(6), 293–327.