| Line 69: | Line 69: | ||
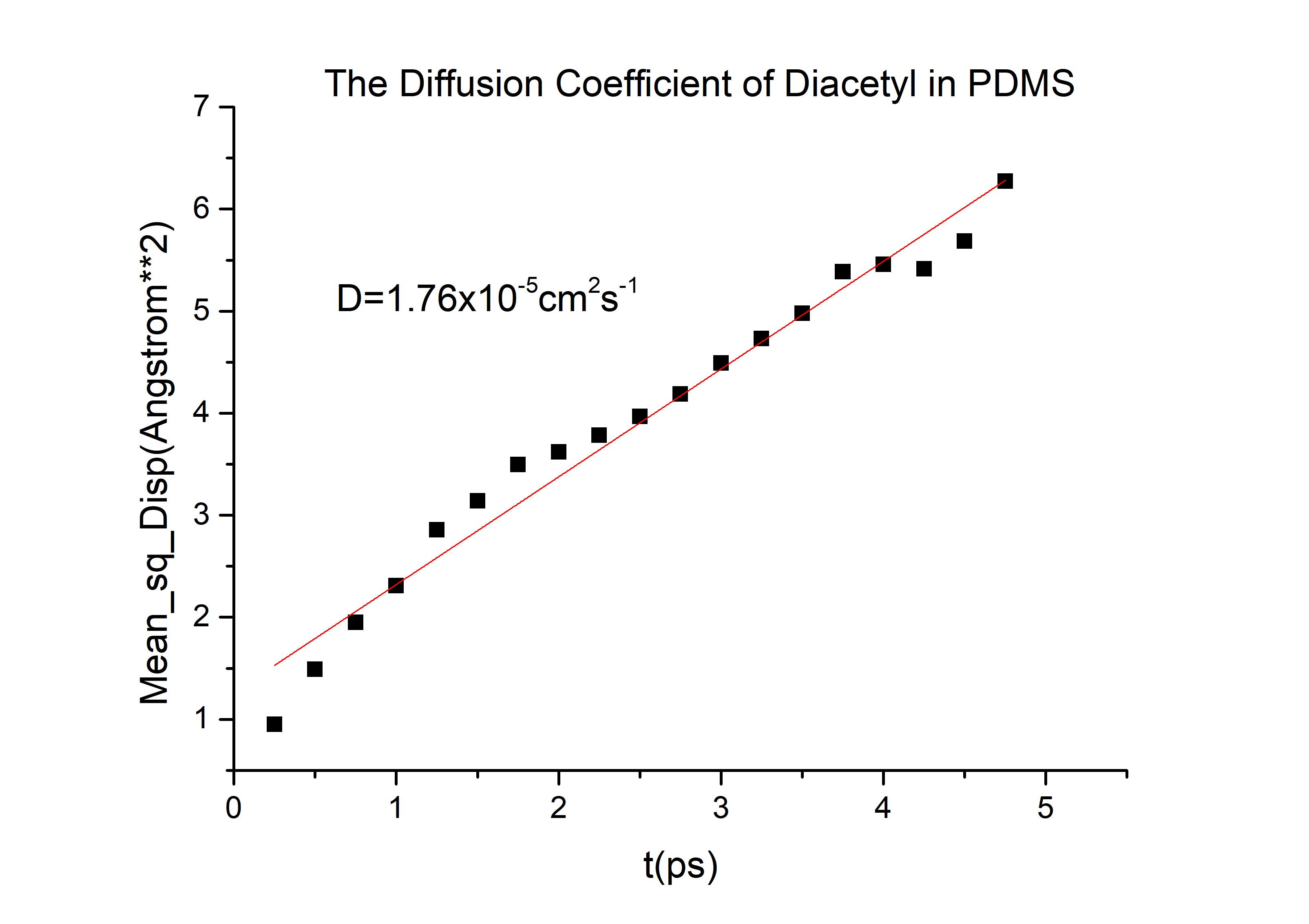
{{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--Microfuildics6-Model.png|width=800px|caption=<B> Fig.1.7 The diffusion coefficient of diacetyl in PDMS</B>}} | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--Microfuildics6-Model.png|width=800px|caption=<B> Fig.1.7 The diffusion coefficient of diacetyl in PDMS</B>}} | ||
| − | = 2. MiniMos | + | = 2. MiniMos Transfection = |
===2.1 Overview === | ===2.1 Overview === | ||
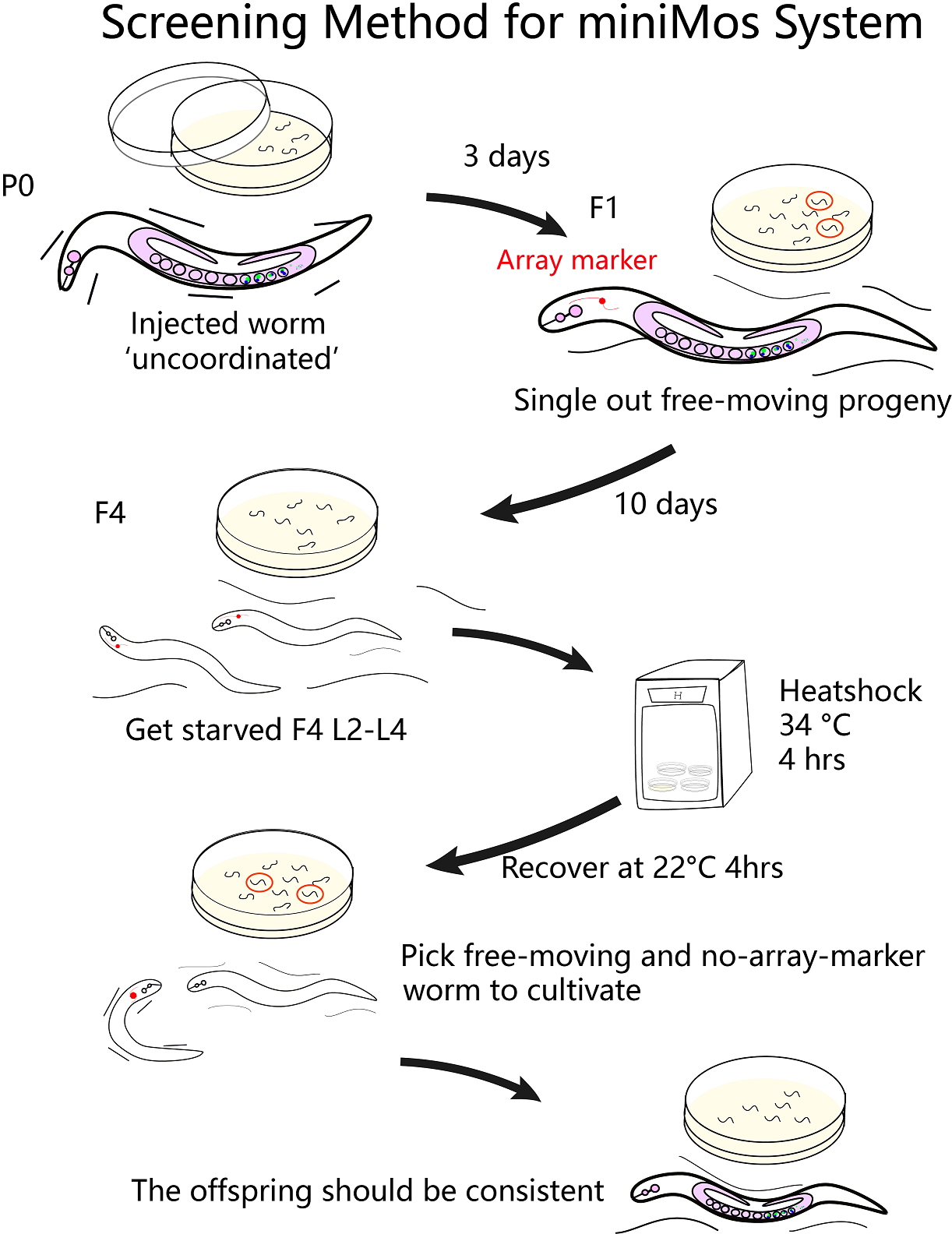
In our program, we aim to change the physical behaviour of C. elegans using the two specific optogenetic traits.Through microinjection and selection, we are able to get two strains with two phenotypes of the preference to blue light and the aversion to red light , and the next step is to obtain the single worm with the combination ofe these 2 different traits. | In our program, we aim to change the physical behaviour of C. elegans using the two specific optogenetic traits.Through microinjection and selection, we are able to get two strains with two phenotypes of the preference to blue light and the aversion to red light , and the next step is to obtain the single worm with the combination ofe these 2 different traits. | ||
| + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--_overviewofmosmodel.png|width=600px|caption=<B>Fig. 2.1 The overview of MiniMos Transfection Model}} | ||
===2.21 The MiniMos successful rate=== | ===2.21 The MiniMos successful rate=== | ||
Revision as of 01:52, 20 October 2017
Model
Project
1. Diffusion
1.1 Overview
Modeling is usually used to make sense of the experimental discovery in traditional biological studies. In this synthetic biology project, we believe that carefully carried out modeling will be critical for the experiment design and data analysis at different stages of the project. We hope to demonstrate that the modeling is especially helpful to finalize the microfluidics chip design, and determine the concentration of the chemicals and the length of time we do the experiments. In our project, we need to prove that the C.elegans will not change their likes and dislikes after we add the plasmids into them. Therefore, we designed the Gaussian plate (Fig.1.1) a microfluidic chip.[1] Using this chip we can know the C.elegans’ likes and dislikes through their distribution in the chip.
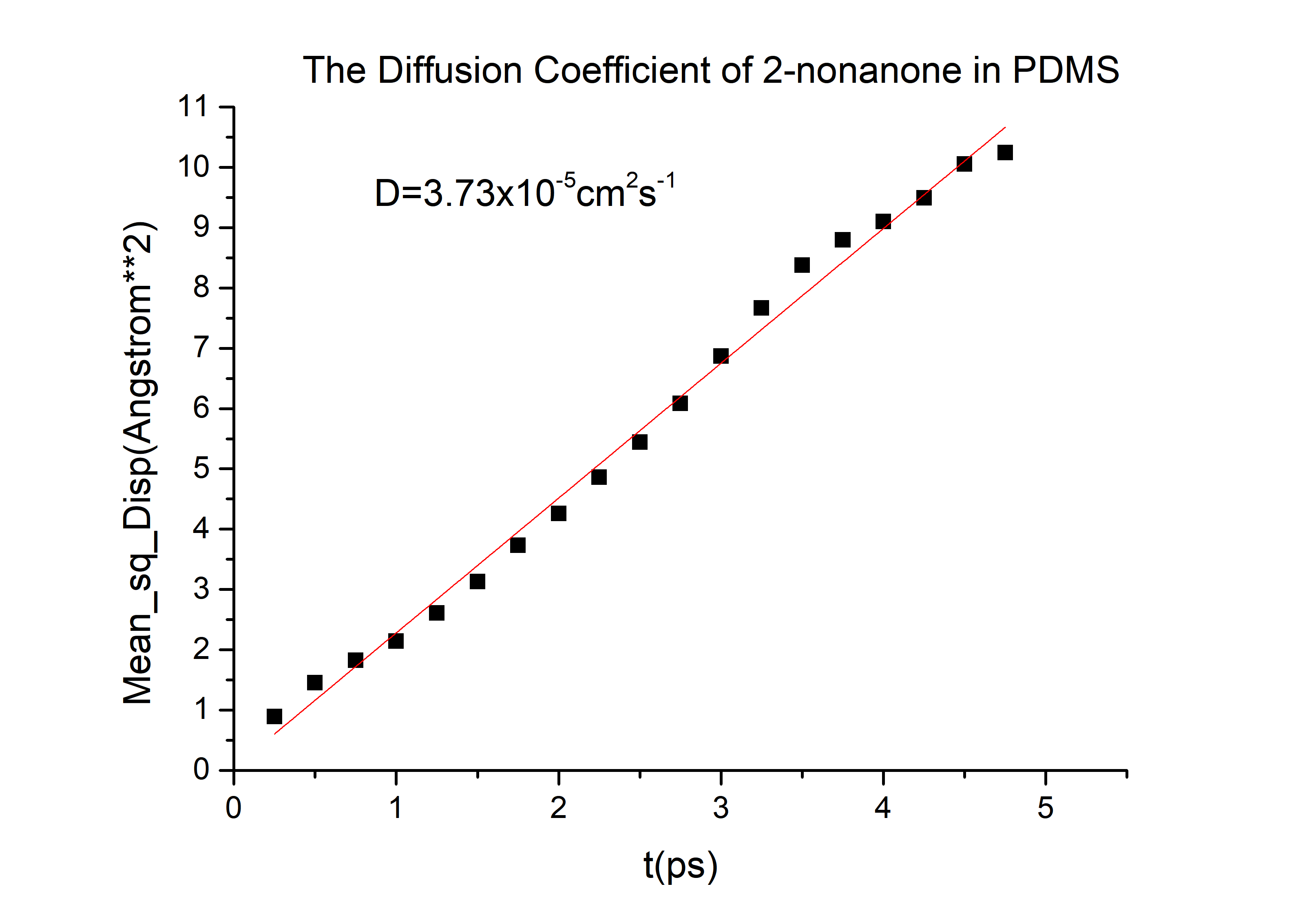
In order to simulate the distribution of the C.elegans we need to know the diffusion of diacetyl (C.elegans prefers it) and 2-nonanone (C.elegans detests it [2]) in Polydimethylsiloxane (PDMS), a kind of transparent and air permeable material used in microfluidics. Therefore, we came up with following modeling. For nonsteady-state diffusion, there is an equation named Fick’s second law[3]: \frac{\partial C}{\partial t}=D \frac{\partial^2 C}{\partial x^2}The constant of proportionality D is called the diffusion coefficient, which is expressed in square meters per second. Concentration C is plotted versus position (or distance) within the solid x and the time t. For t=0, C=C0 at 0≤x≤∞; For t>0, C=Cs (the constant surface concentration) at x=0;C=C0 at x=∞. And then there is a general solution: \frac{C_x-C_0}{C_s-C_0}=1-erf(\frac{x}{2 \sqrt{Dt}}) Where Cx represents the concentration at distance x after time t.
Because\frac{x}{2 \sqrt{Dt}}=constant,so if this constant can be calculated, the equation of distance x and time t can also be gotten.
In our model, C0=0, so the equation is changed to \frac{C_x}{C_s}=1-erf(\frac{x}{2 \sqrt{Dt}}) Cx represents the concentration at distance x that C.elegans can feel;Cs is the concentration set by our own and it is a constant.x is from 0mm to 0.05mm.
C(2-nonanone) = 1*10-2 mol/L. C(diacetyl) = 2.8*10-3 mol/L (diacetyl:M9 buffer=1:350)
And D for 2-nonanone and diacetyl in PDMS. We need to calculate them.
1.2 The Calculation of Diffusion Coefficient(D)
The diffusivity of a gas in an organic solvent, polymer, or zeolite can be calculated by running a molecular dynamics simulation and determining the mean square displacement of the gas in the material. This allows us to calculate the self-diffusivity coefficient of the gas and gives an insight into the overall diffusivity. As we are performing a molecular dynamics calculation, we can analyze the effect of temperature, pressure, density, and penetrant size and structure on diffusion. We used force-field method to calculate the D in PDMS by Material Studio (MS) a software for material calculation.[4][5]
1.2.1 Set up the initial structures
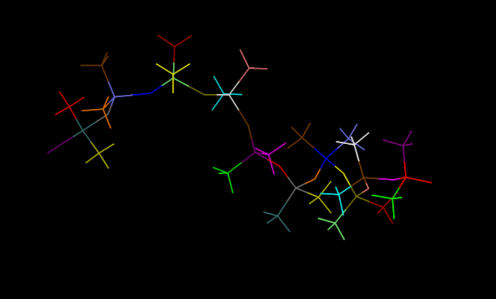
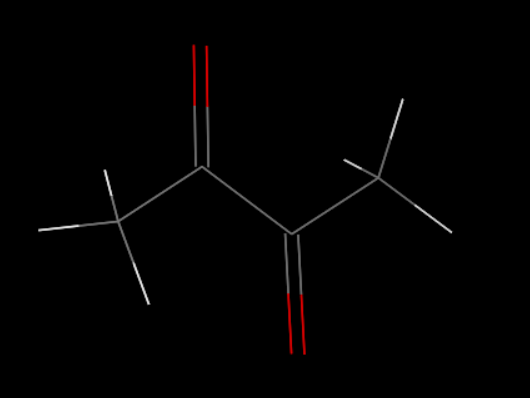
First, we set up the initial structure of the PDMS and the chemicals (Fig.1.2 and Fig.1.3).
1.2.2 Build an amorphous cell
Then, we put the two molecules in to an amorphous cell (Fig.1.4).
1.2.3 Relax the cell
When we generate an amorphous cell, the molecules may not be equally distributed throughout the cell, creating areas of vacuum. To correct this, we must perform a short energy minimization to optimize the cell. After the minimization, we should run a short molecular dynamics simulation to equilibrate the cell.
1.2.4 Run and analyze molecular dynamics
1.2.5 Export data and calculate the diffusivity
Then we can got the D of 2-nonanone and diacetyl:
D(2-nonanone) = 6.78*10-6cm2/s D(diacetyl) = 6.78*10-6cm2/s
2. MiniMos Transfection
2.1 Overview
In our program, we aim to change the physical behaviour of C. elegans using the two specific optogenetic traits.Through microinjection and selection, we are able to get two strains with two phenotypes of the preference to blue light and the aversion to red light , and the next step is to obtain the single worm with the combination ofe these 2 different traits.
2.21 The MiniMos successful rate
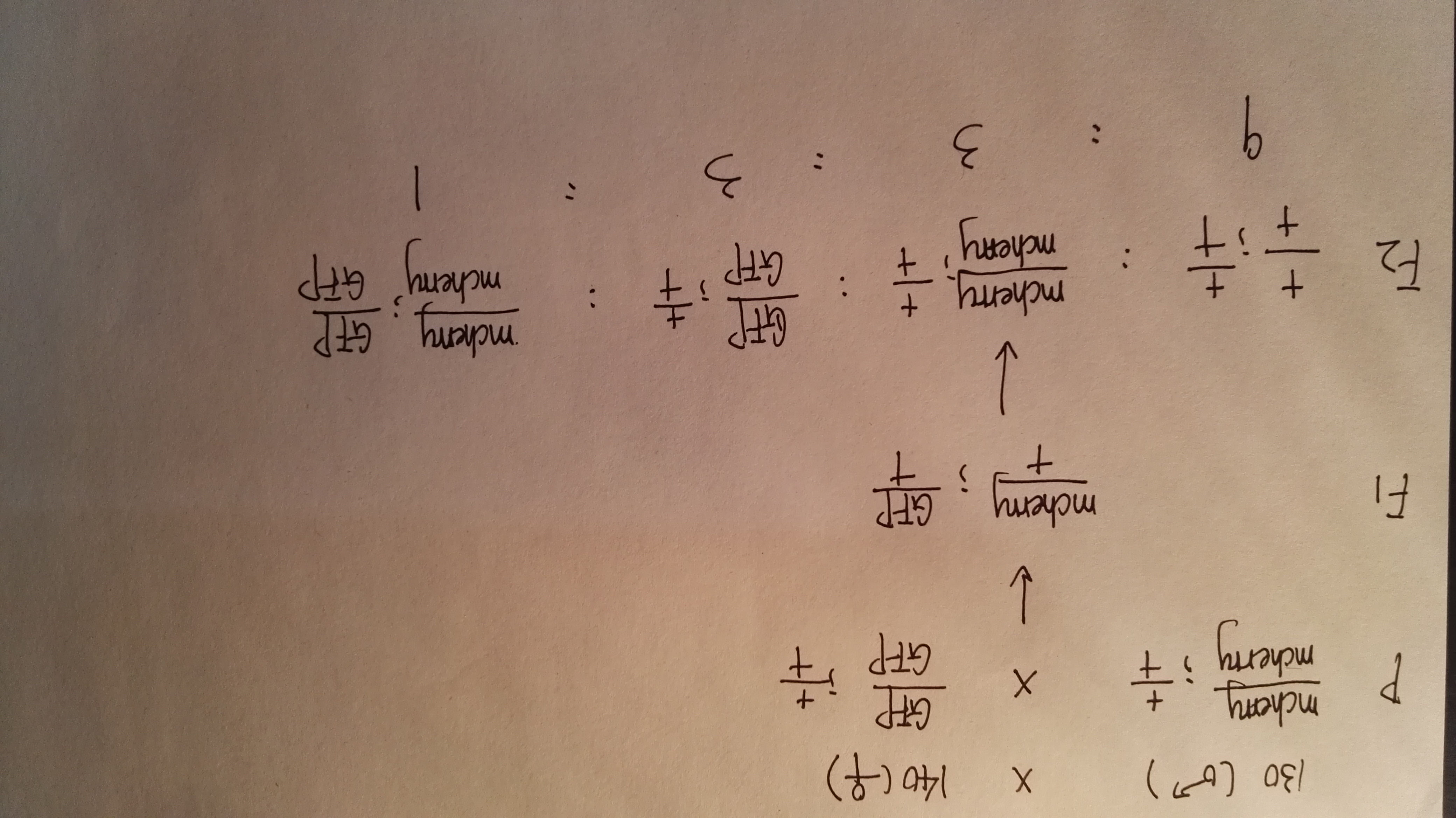
First considering the successl rate of miniMos system, the probability of correct injection is approximately 60% due to manual errors, and about 10% of F1 offsprings are all rescued, meaning that the plasmid expression rate(transposon transfer target gene into the chromosome) is about 10%. So the successful rate is 60% * 10% = 6%.
2.22 The stable transfection rate
Then, during the F2 generation, for two genes on different chromosomes, we can calculate the possiblity of target strains in F2 by Mendel's laws. The prospecting result is 1/16. Therefore, the overall probability to get the target strain is about 60% * 10% * (1/16) *100% = 0.375%.
===2.23 The effect of crossing over
However, this probability does not consider the chance of crossing over. Accroding to the formula, the rate of crossing over is (the number of recombinant genotype offsprings)/(the number of total offsprings)*100%. Therefore, the possible rate of crossing over in our experiment is 10/354 = 2.8%.
So the final rate is 0.375% * (1 - 2.8%) = 0.3645%.
References
- ↑ Albrecht, D. R. and C. I. Bargmann (2011). “High-content behavioral analysis of Caenorhabditis elegans in precise spatiotemporal chemical environments.” Nature Methods 8(7): 599-605.
- ↑ Troemel, E. R., Kimmel, B. E., & Bargmann, C. I. (1997). Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in c. elegans. Cell, 91(2), 161-9.
- ↑ Callister, W. D., & Rethwisch, D. G. (2004). Fundamentals of Materials Science and Engineering. John Wiley and Sons Ltd.
- ↑ S. G. Charati† and, & Stern, S. A. (1998). Diffusion of gases in silicone polymers: molecular dynamics simulations. Macromolecules, 31(16), 5529-5535.
- ↑ Hofmann, D., Fritz, L., Ulbrich, J., Schepers, C., & Böhning, M. (2000). Detailed‐atomistic molecular modeling of small molecule diffusion and solution processes in polymeric membrane materials. Macromolecular Theory & Simulations, 9(6), 293–327.