Results
Project
Contents
Microfluidics were divide into three parts: the Selection Chip; the Gaussian Plate; and the Immobilization Chip.
The Selection of the Caenorhabditis elegans
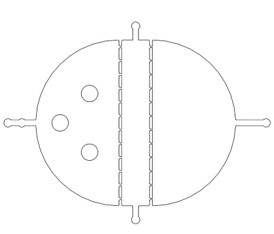
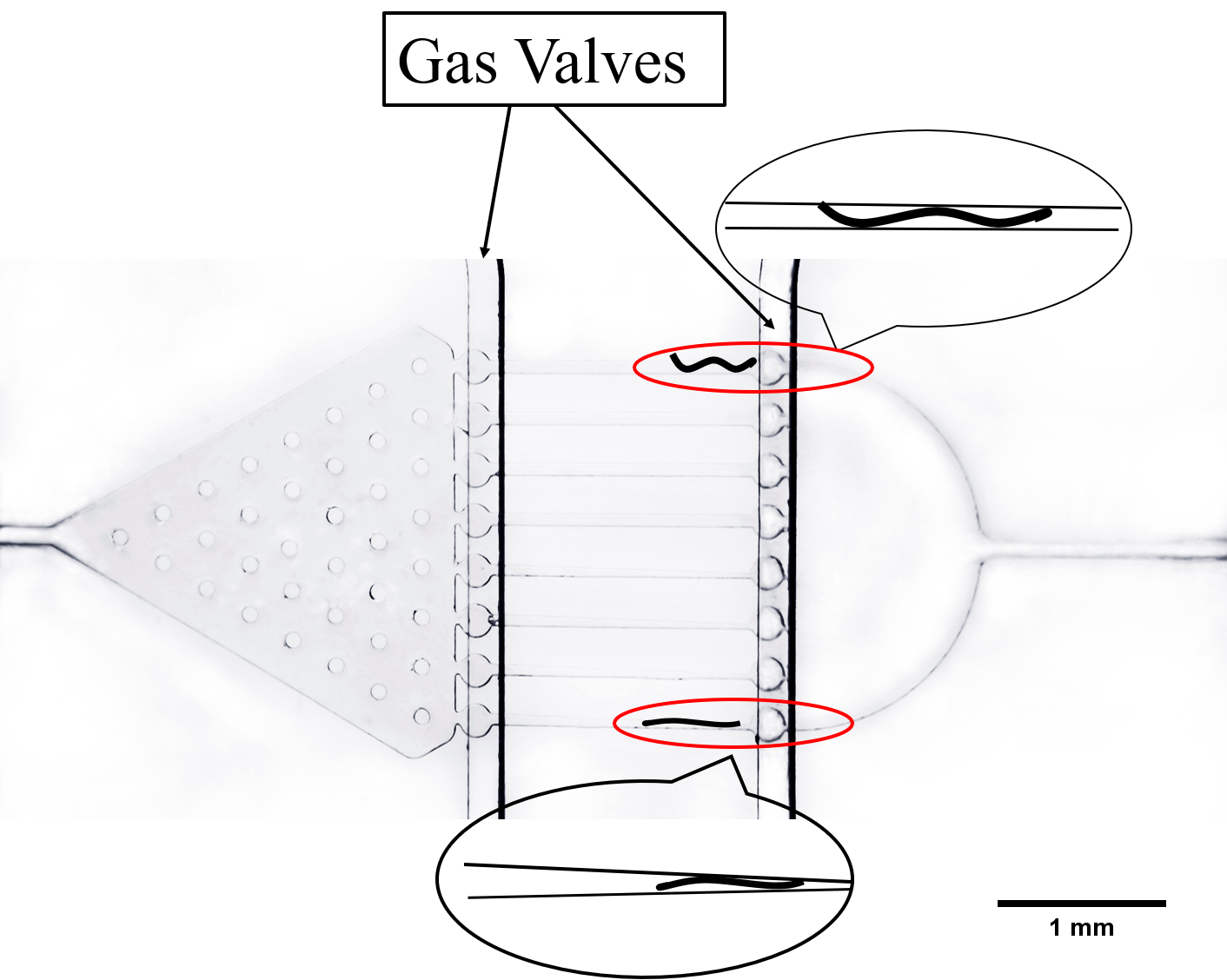
There are two plans of selecting worms. Why we need the C. elegans with the same stage The first one is using microfluidics. We designed the Selection Chip to select the Caenorhabditis elegans (C. elegans) with the appropriate size (Fig.1).
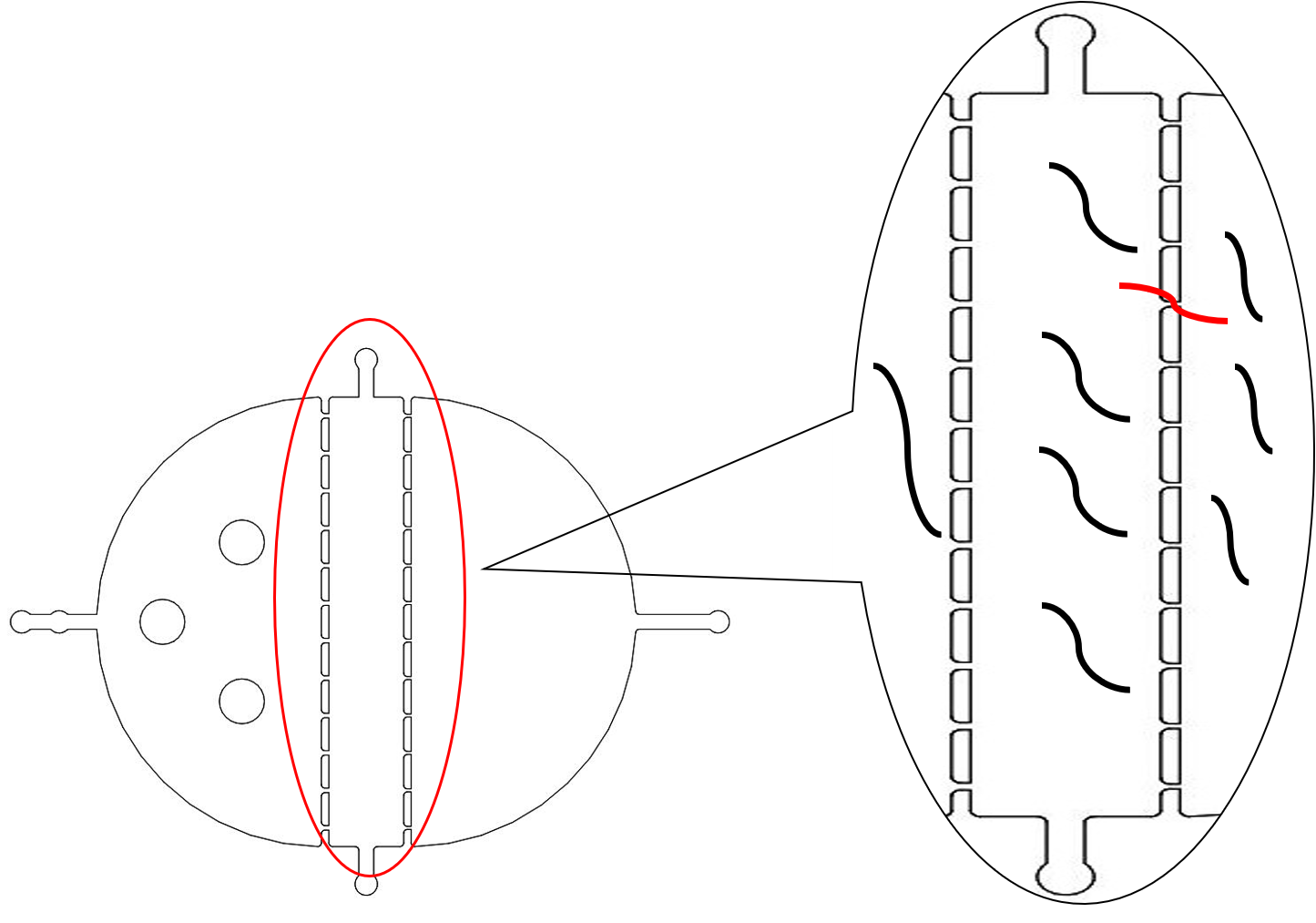
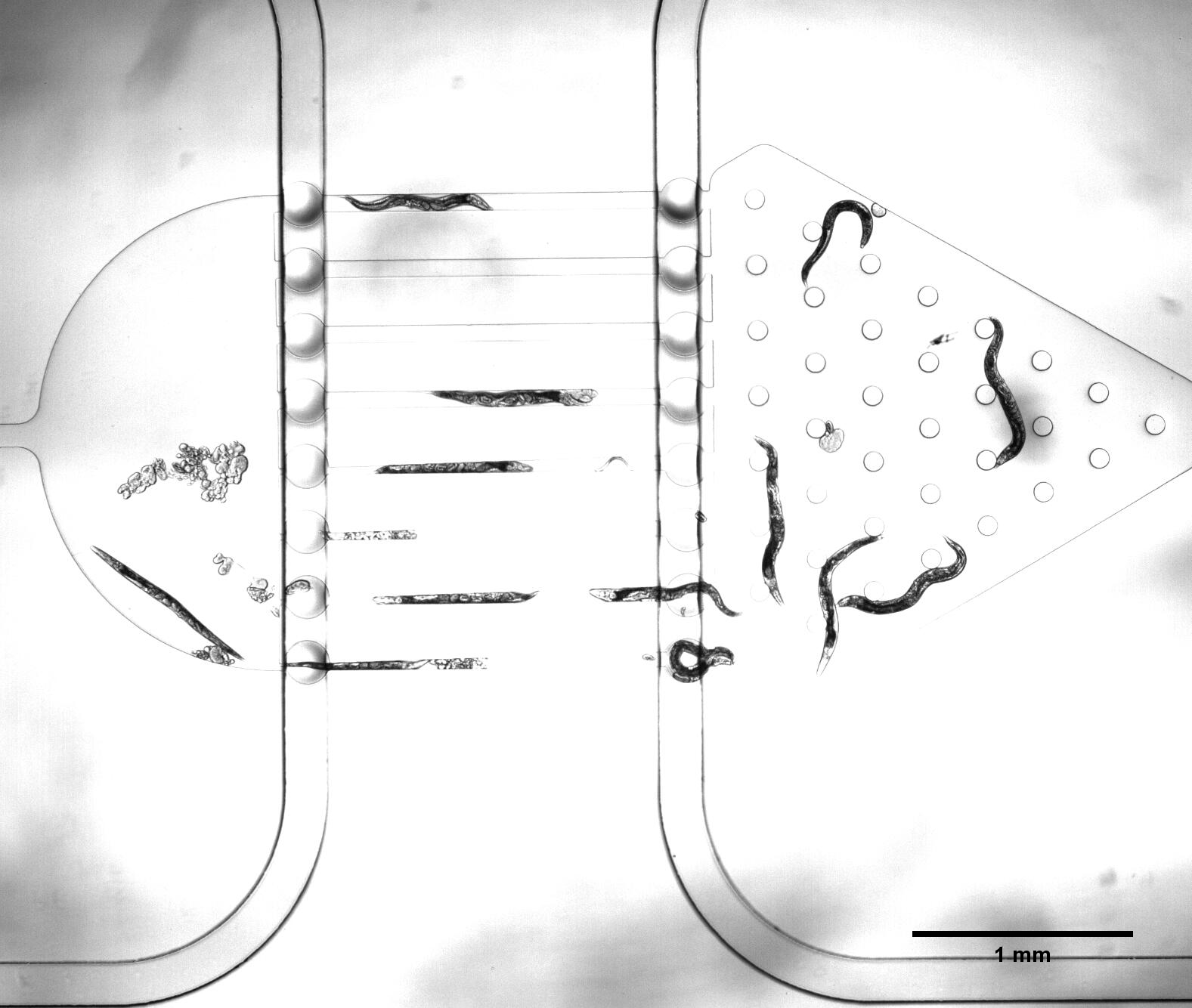
We need a large number of the worms with the same stage to do the Gaussion Plate. Why we need so many with the same stage However, we found that the chip only has 12 fences (Fig.2) The efficiency of the Selection Chip was very low because of such a small number of the fences. In addition, the C. elegans have flexible body, some of the suitable size worms would still go through the second fences (Fig.2).
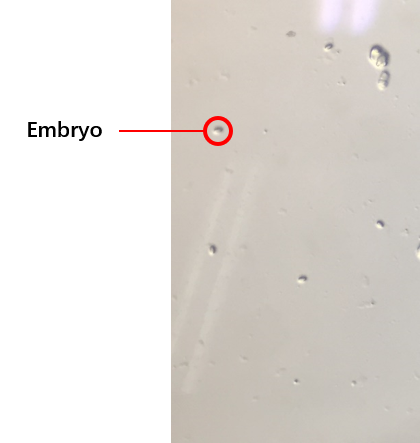
The second plan was the C. elegans’ synchronization.[1](How to do the C. elegans’ synchronization ) We got the embryos (Fig.3) from the old worms so that the worms would be at the same stage because of the hatches of the embryos were at the same time. We selected several conditions of the synchronization, finally, we could get the worms at the same stage. The synchronous rate:
\frac{N_1*100\%}{N_2}
N1 equals the number of worms at L4
N2 equals the number of all worms.
can reach to about 80%.
The Gaussian Plate
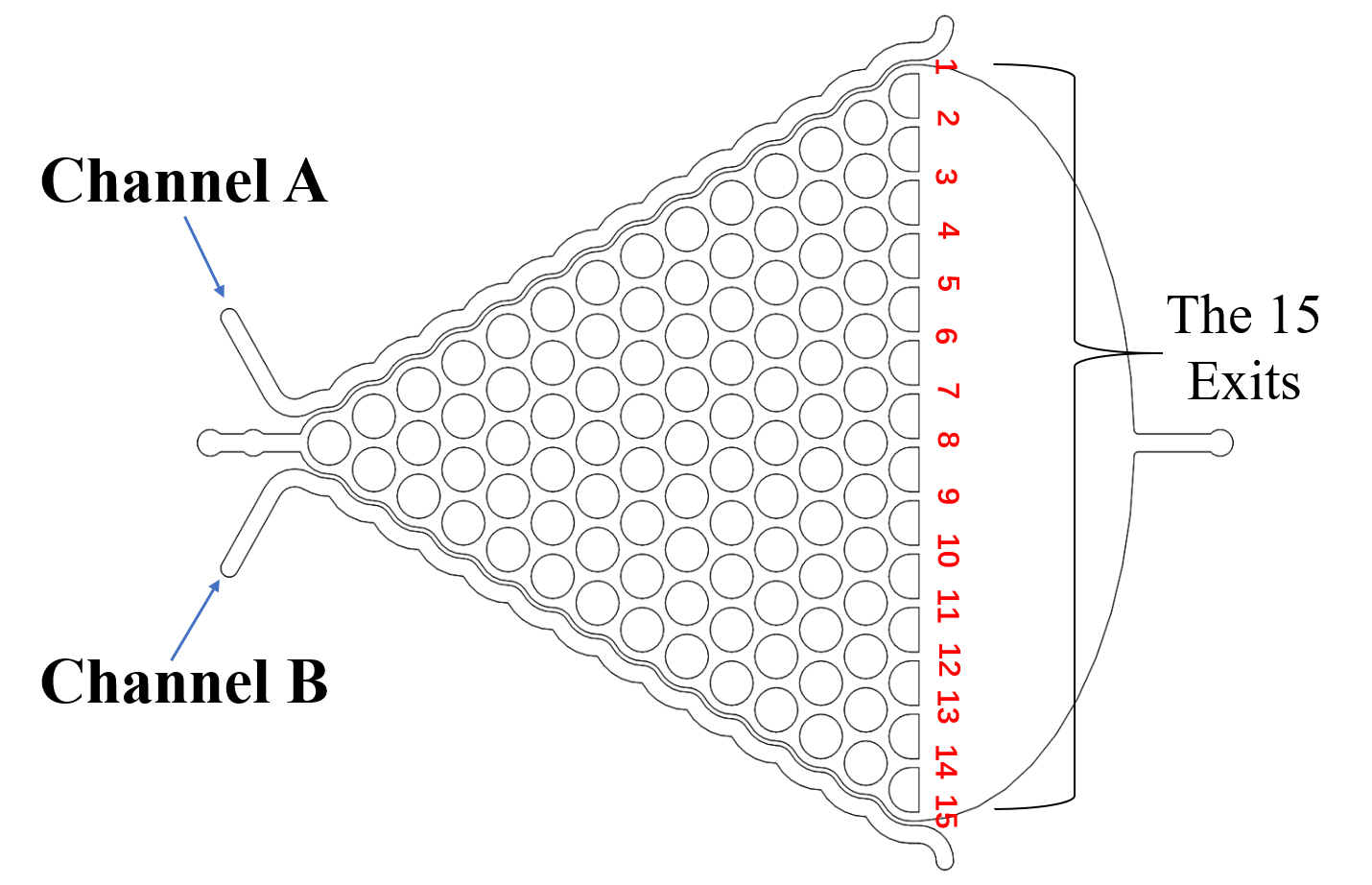
The Gaussian Plate (Fig.4)[2] was designed to test if our exogenous genes would influence their olfactory receptor neuron pair (preference and repulsion to some chemical odors).
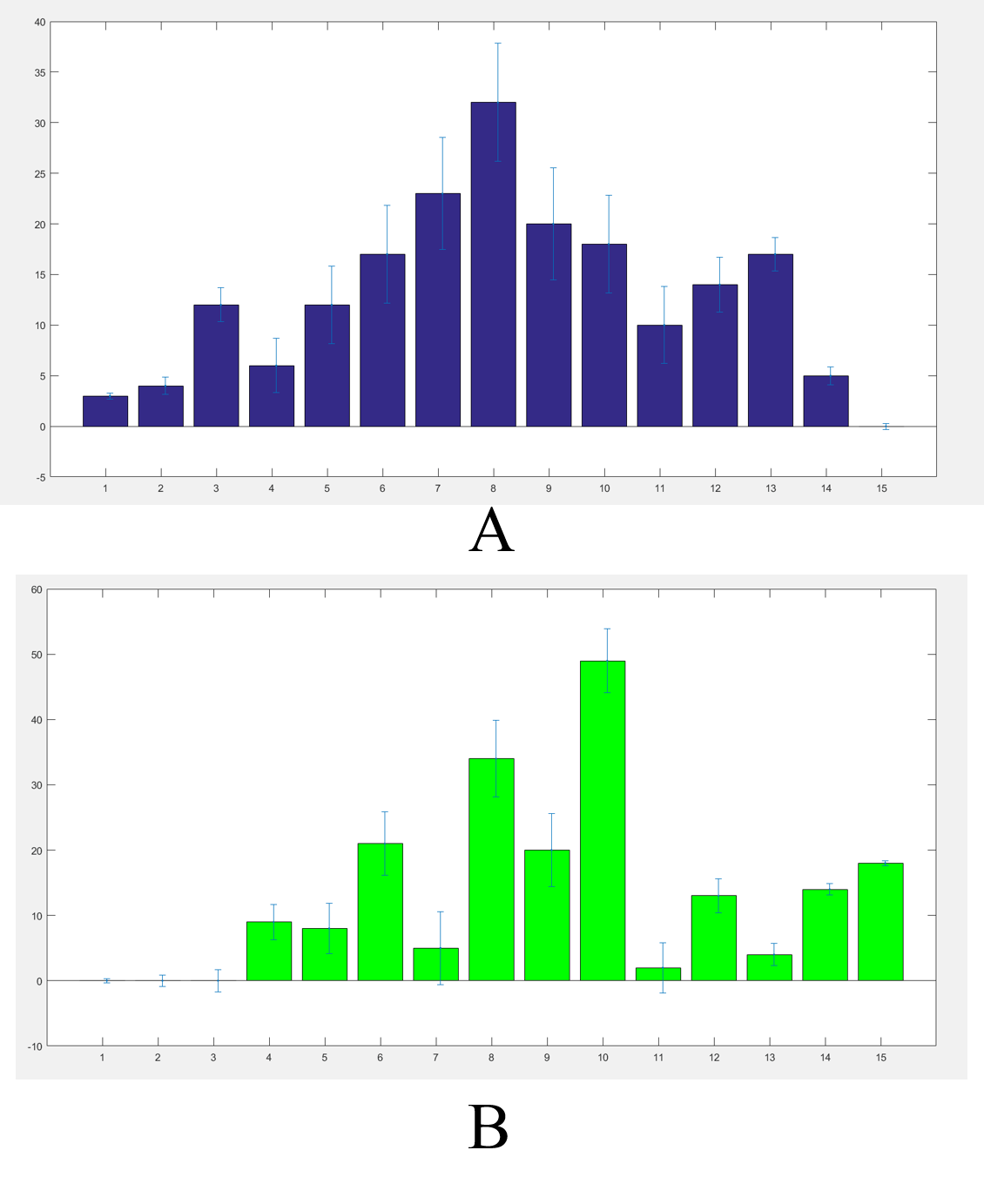
We got the worms’ distributions(Fig.5) after several experiments for the wild type worms and our experimental worms with or without the chemicals (Fig.6).
The final result were not such a good Gaussian distribution like the Galton board because the C. elegans' choices were not absolutely normal. In order to adjust our results we built a model.(the worm locomotion model)
The Immobilization Chip
The immobilization Chip was deigned to immobilize the C. elegans in worm traps or parallel channels for worm imaging and ethological experiments.[3]
We could immobilize the worms in the worm traps (Fig.8) and watch the neuronal activity (Fig.9) successfully using fluorescence microscope (Nikon eclipse Ti).
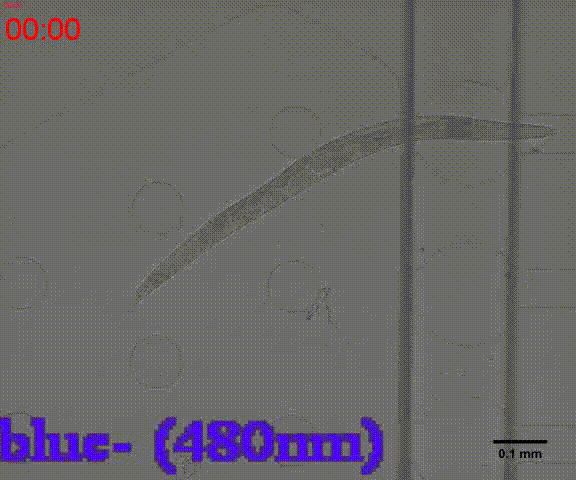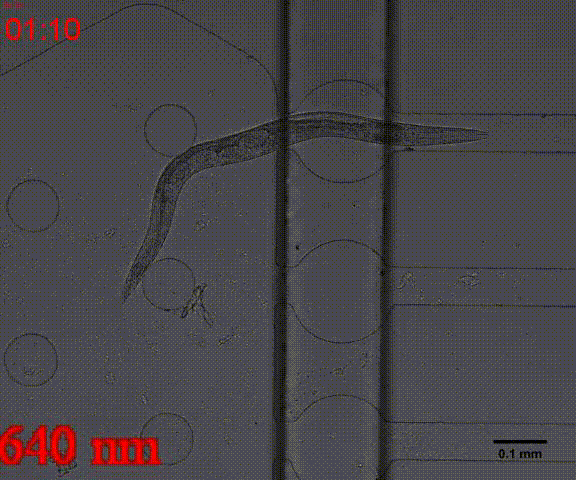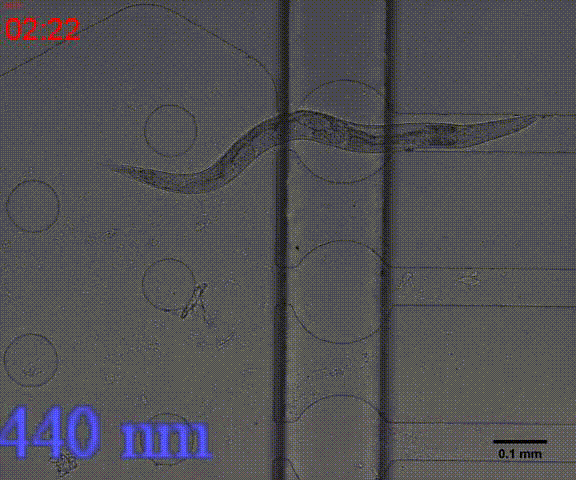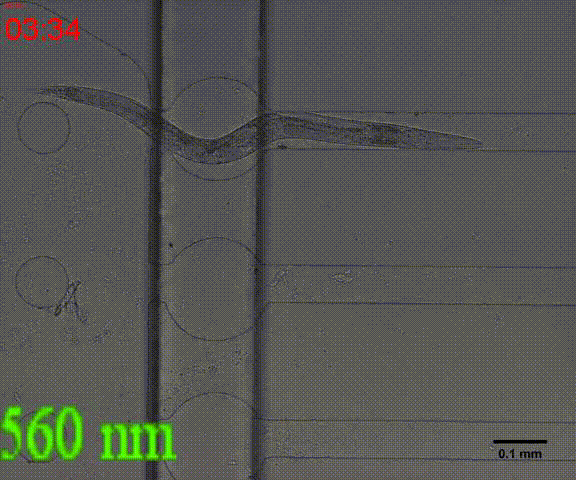
In addition, we could stimulate the Odr10::CoChR::GEM-GECO::mCherry worms to be active from the low state (Fig.10). On the other hand, we could also get the excitation wavelength of CoChR. The result showed that the light from the projector without filter (OD8) and the lights with 395 and 440 wavelengths from the LED of fluorescence microscope (Nikon eclipse Ti) could influence the C. elegans the other lights cannot. The lights with 395 and 440 wavelengths are closed to the ultraviolet which would hurt the C. elegans, so we got that the lights from the projector without the filter could active the CoChR. Hardware of light
Unfortunately, we cannot see the clear neuro activity in str1::Chrimson::GEM-GECO::GFP worms using fluorescence microscope but we can use confocal microscope to observe the neuronal successfully.
References
- ↑ Portadelariva, M., Fontrodona, L., Villanueva, A., & Cerón, J. (2012). Basic caenorhabditis elegans methods: synchronization and observation. Journal of Visualized Experiments Jove, 64(64), e4019.
- ↑ Albrecht, D. R. and C. I. Bargmann (2011). “High-content behavioral analysis of Caenorhabditis elegans in precise spatiotemporal chemical environments.” Nature Methods 8(7): 599-605.
- ↑ San-Miguel, A., & Lu, H. (2013). Microfluidics as a tool for C. elegans research. Wormbook the Online Review of C Elegans Biology, 1.