| Line 56: | Line 56: | ||
{{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--3.gif|width=6000px|caption=<B>Fig. 6 C.elegans' locomotion in the Gaussian plate</B> C.elegans can move forward freely in the channel, and then we will get its locomotion distribution just like the Galton Nail Plate model. However, after adding diacetyl in the side channel, the distribution is changed. C.elegans tend to move towards the right (or the left) due to their preference to diacetyl.}} | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--3.gif|width=6000px|caption=<B>Fig. 6 C.elegans' locomotion in the Gaussian plate</B> C.elegans can move forward freely in the channel, and then we will get its locomotion distribution just like the Galton Nail Plate model. However, after adding diacetyl in the side channel, the distribution is changed. C.elegans tend to move towards the right (or the left) due to their preference to diacetyl.}} | ||
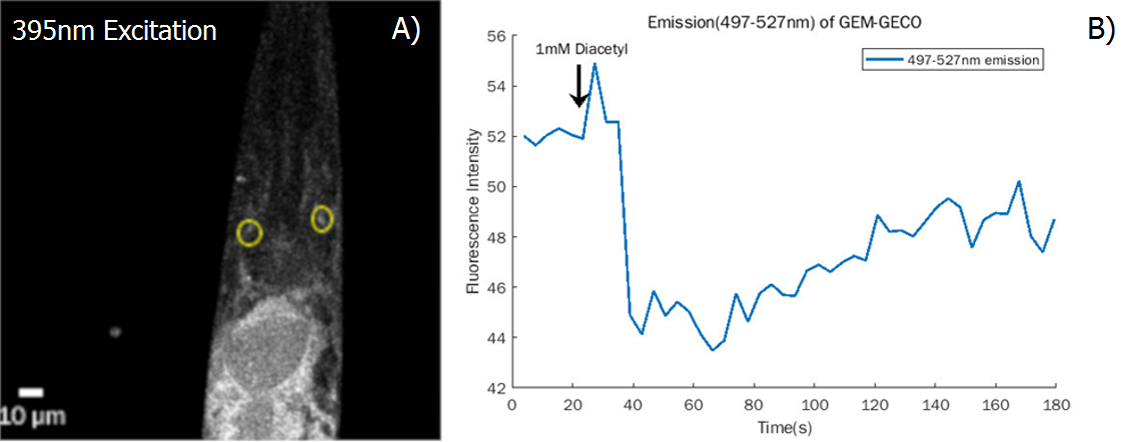
| − | {{SUSTech_Image_Center_fill-width | filename=T--SUSTech_Shenzhen--Demonstrate-fig7.png|width=6000px|caption=<B>Fig.7 Demonstrate the function of AWA neuron A) Confocal Image of worm’s head</B> neuron cells of AWA neurons are circled at the photo (yellow) <B>B) The fluorescence intensity of 497-527nm emission of GEM-GECO falls steeply after applying diacetyl.</B>}} | + | {{SUSTech_Image_Center_fill-width | filename=T--SUSTech_Shenzhen--Demonstrate-fig7.png|width=6000px|caption=<B>Fig.7 Demonstrate the function of AWA neuron A) Confocal Image of worm’s head</B> neuron cells of AWA neurons are circled at the photo (yellow) <B>B) The fluorescence intensity of 497-527nm emission of GEM-GECO falls steeply after applying diacetyl.</B> Combined with calcium, the emission wavelength of GEM-GECO shift from 510nm to 470nm ( central peak)}} |
Revision as of 03:43, 2 November 2017
Demonstrate
Project
Contents
In our project, we want to study the neural network activity and behavioral response of Caenorhabditis elegans with orthogonal optogenetic stimulation of two neurons.
So far, we have achieved almost all of our project objectives, building a platform to study the learning behavior of Caenorhabditis elegans. The whole project is demonstrated in the following parts:
- Choosing the suitable excitation wavelength of light-sensitive proteins
- Genetic integration of synthetic circuits into C.elegans strains
- Examining the functions of AWA and AWB neurons
- Using Microfluidic device to examine the behavioral change of individual worms
- Training C.elegans to be addicted to alcohol
Choosing the suitable excitation wavelength
Expression of two types of channelrhodopsins in two pairs of receptor neurons enables worm to be attracted by blue light and be repelled by red light. At the same time, we plan to use GEM-GECO as calcium indicator to examining the neuron activation, and perform feedback control in the future.
To avoid potential crosstalk, we need to separate the excitation wavelengths of CoChR activation and GEM-GECO imaging. In our project, we chose 395nm and 470nm to activate CoChR and GEM-GECO.
Before the experiment in worms, we conducted a validation experiment on CHO-K1 cell line expressing CoChR to identify a suitable excitation wavelength for the light-sensitive ion channel.
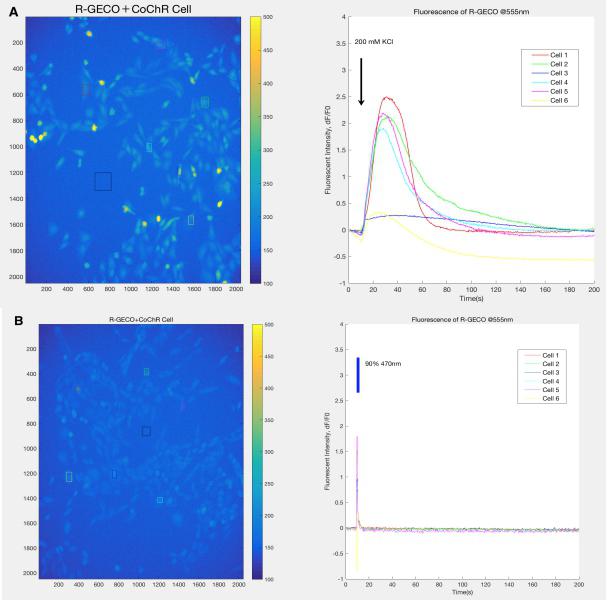
Based on the result, we determine to use 470nm to activate the CoChR and 395nm for the excitation of GEM-GECO.
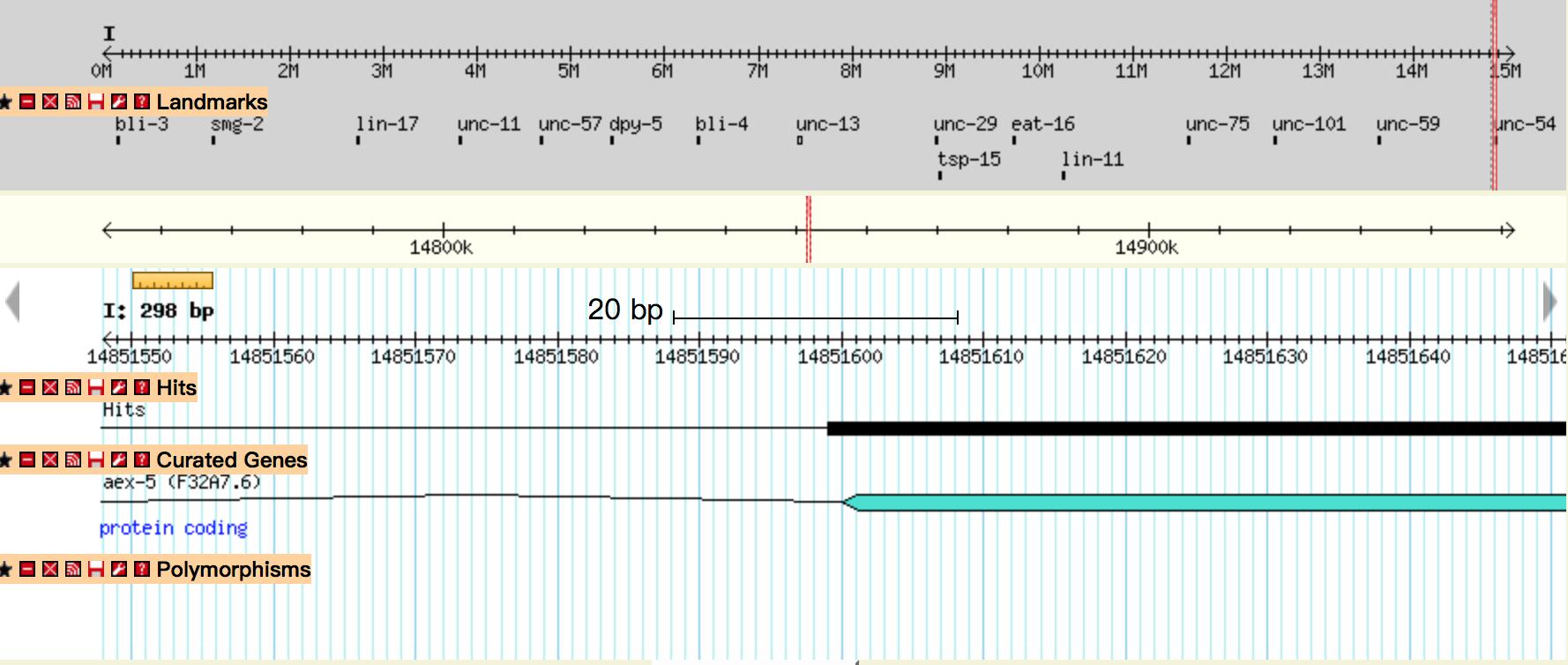
Genetic integration of synthetic circuits into C.elegans strains
We used the miniMos system to integrate the gene circuits into C.elegans genome. After injection and selection (Fig.2), we successfully obtained the inserted worms (Odr-10::CoChR::GEM-GECO::mCherry and str-1::Chrimson::GEM-GECO::GFP) .
After getting the genetically inserted worms, we successfully got the hybrid by mating 2 strains.
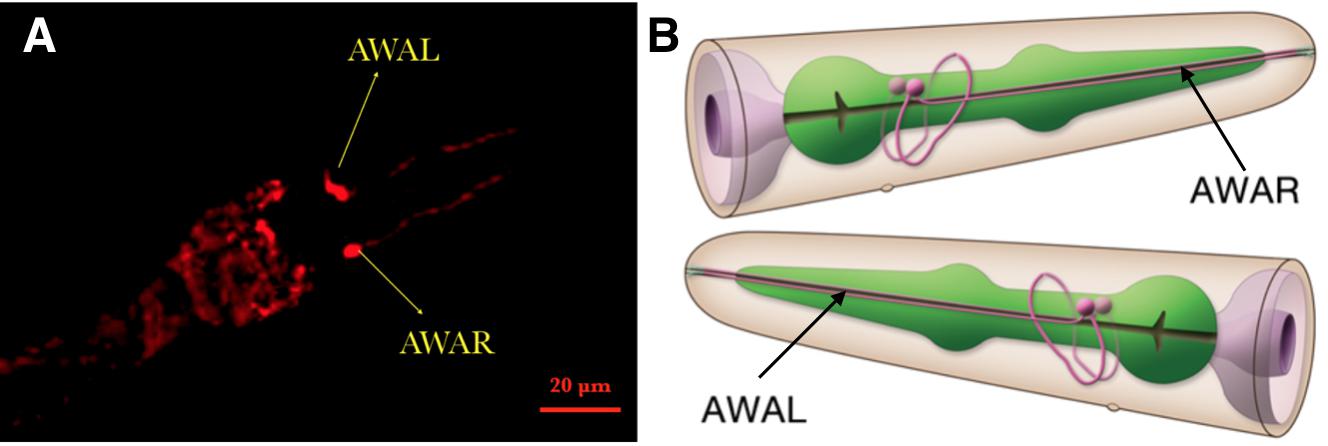
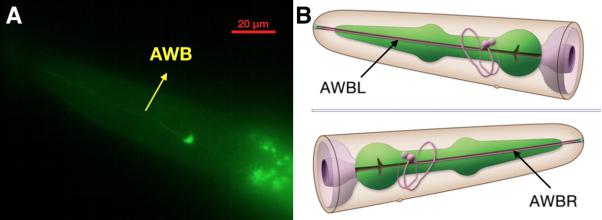
Examining the function of AWA and AWB neurons
Firstly, the experiment in which inserted worms are strongly attracted by diacetyl shows that the insertion did not degrade normal neuron function (Fig.6)
We also test the result in the neuron level (Fig.7)

Examining the behavioral change of individual worms
We designed two methods to observe inserted worms’ behavior change:
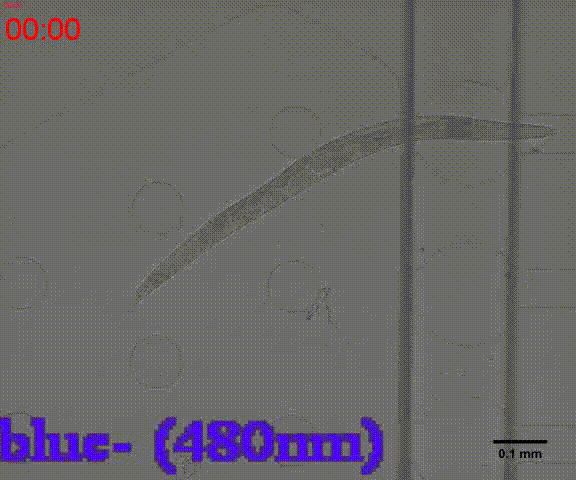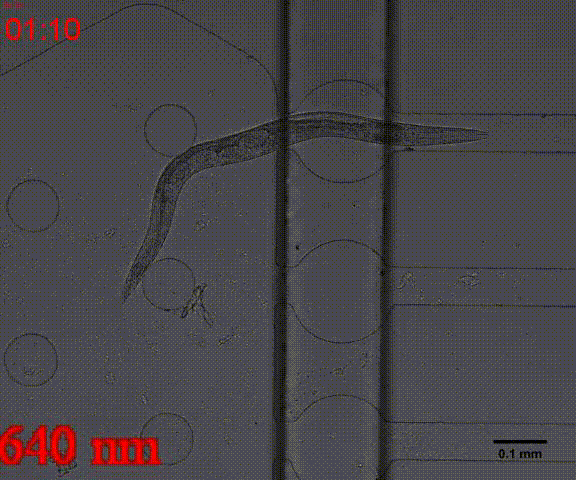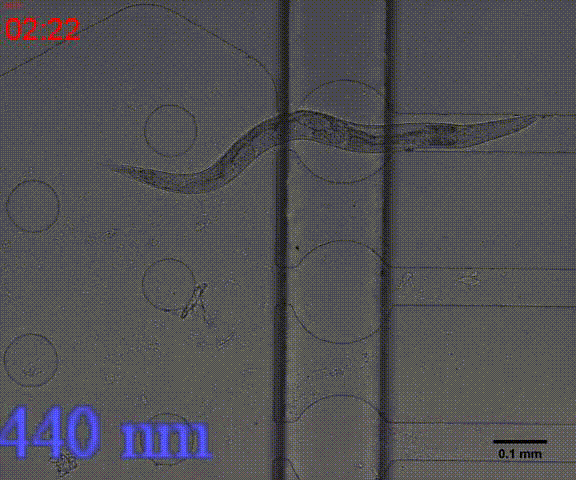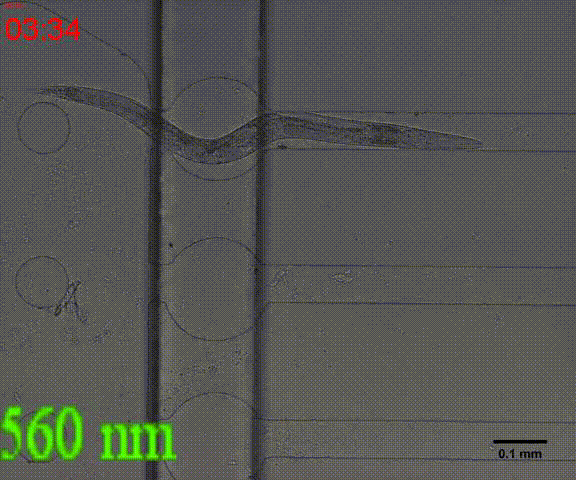
- Inducing free-moving worms by certain wavelength (Fig.7)
- Stimulating worms semi-fixed in microfluidic chips (Fig.8)
In the inducing experiments, Odr-10::CoCHR::GEM-GECO::mCherry worms shows a strong preference to blue light. In the later experiment, we will use this preference to train them to form specific behavior.
Unfortunately, str-1::Chrimson::GEM-GECO::GFP worms are not clearly repelled by the red light. The low sensitivity is probably due to the expression level of Chrimson under the control of str-1 promoter. An modified circuit containing amplification step is design, but the implementation cannot meet the deadline of this wiki.
Fig. 7 Behavior experiments of C.elegans. These two figures show the worm have obvious response to the blue light which could activate channelrhodopsin CoChR. the first video shows the worm which expressed Odr-10::CoChR::GEM-GECO::mCherry circuit could run in circle to follow the blue light. The other figure shows the same type worm which looks “asleep” ( because of no food supply) be “waked up” also by blue light(preference).
Alcohol addiction
We trained the worms engineered with Odr-10::CoChR::GEM-GECO::mCherry to be “addicted to” alcohol successfully.
- In this experiment, we added the alcohol on the NGM plate containing engineered worms. At the same time, blue light is turned on to stimulate worms continually.
- After training for 2 hours, we washed the plate and recovered the worms in M9 buffer.
- Then, we put the mixture contained worms and M9 buffer on one side of a new plate. After recovery of the worms, we added the alcohol on the other side of the same plate to see if the worms have the tropism to the alcohol.
The result shows that most of the worms transfected with Odr-10::CoChR::GEM-GECO::mCherry moved to the alcohol while the wild types could not (Fig.9).
What’s more, without the previous training by light, the worms transfected with Odr-10::CoChR::GEM-GECO::mCherry show no preference to alcohol, neither. (Fig.10).
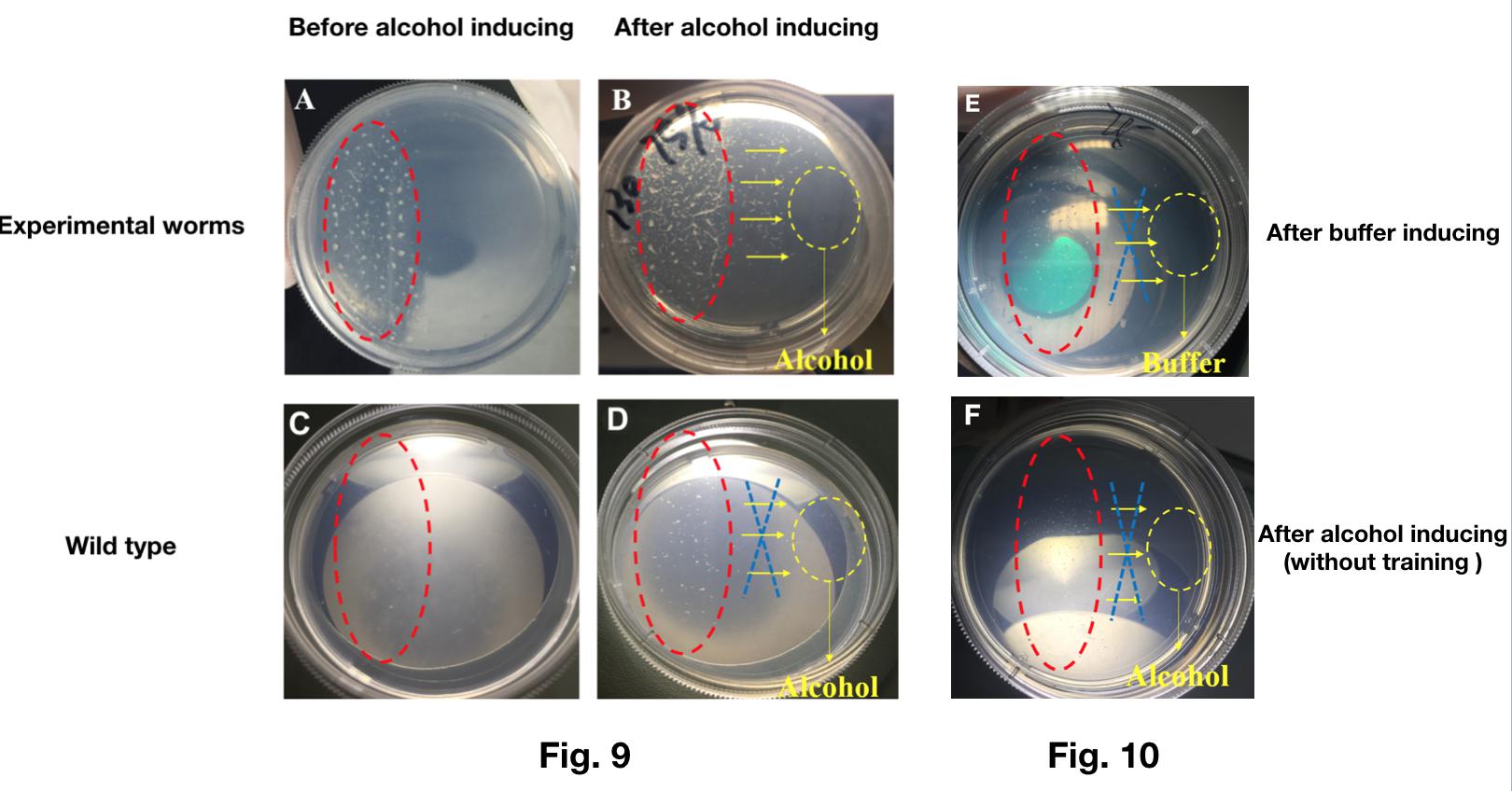
This experiment showed that our genetically modified worms can learn new behavior(addiction to alcohol) after trained by blue light.
Future Plan
Our further work is to decipher the change of neuron connectivity due to the newly learned behavior.
We have selected 2 promoters(cho-1 and unc-8) to express optogenetic circuits on the neurons connected to AWA and AWB, such as AIA, AIY, VB, ASH, AVB, VB, AVA.See details The next step will be using optofluidics platform above to analyze the activation of downstream neurons in the new-formed or modulated neural circuit, with or without the aforementioned training.