| Line 120: | Line 120: | ||
| − | {{SUSTech_Shenzhen/bmath|equ=<nowiki> \left[\begin{matrix}x_{projector}\\y_{projector}\end{matrix}\right]=[\begin{matrix}1&\frac{1024}{L}\end{matrix}](\left[\begin{matrix}x_1\\y_1\end{matrix}\right] | + | {{SUSTech_Shenzhen/bmath|equ=<nowiki> \left[\begin{matrix}x_{projector}\\y_{projector}\end{matrix}\right]=[\begin{matrix}{1}&{\frac{1024}{L}}\\ \end{matrix}](\left[\begin{matrix}x_1\\y_1\end{matrix}\right]\left[\begin{matrix}x_0\\y_0\end{matrix}\right])</nowiki>}} |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
Revision as of 11:12, 31 October 2017
Light Modulator
Let there be light!
Contents
- Weight: 10kg
- Consume: 200W
- Cost: ¥15299.36
Multiple devices of optics are designed and created for the various requirement of experiments. All devices are attempt to modulate the spatio-temporal pattern in a elegant and effective way.
Projector Light Source
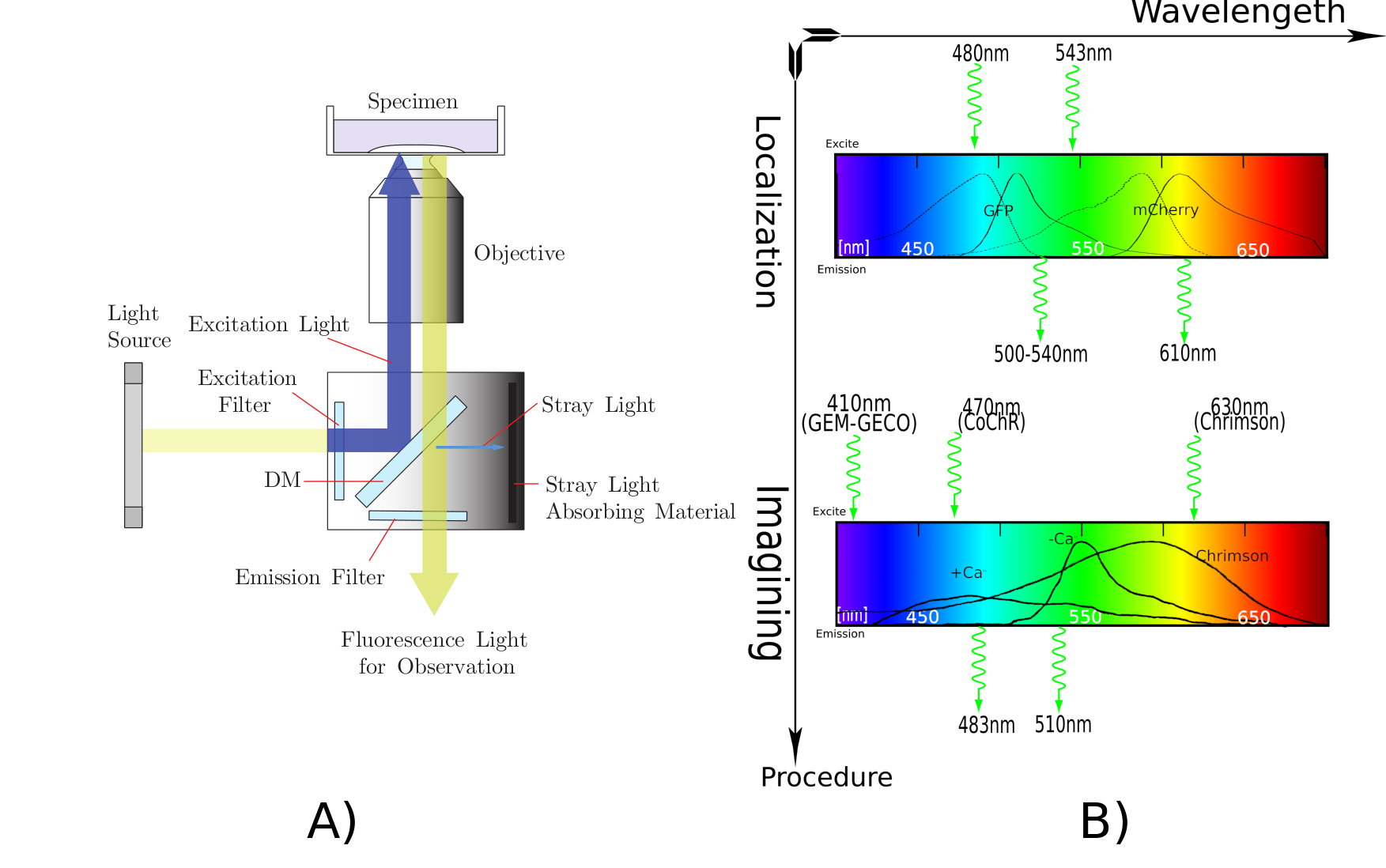
Fluorescence microscope play a important role during our experiment. Zeiss create a perfect visual animation to show the principle of fluorescence microscope, you can find in their [http://zeiss-campus.magnet.fsu.edu/tutorials/basics/axioobserver/indexflash.html web]. However, typical fluorescence microscope can only illuminate the specimen( C. elegans) with same light in whole view. There, we modify a consumed projector to new light source, which can illuminate specimen locally, inmulti color, in multi-points and dynamically. Projector Light Source also has short response time and high light intensity, which will be a effective, hackable and potential tool for synthesis biology.
Optical Principle
Biology background: We need 395nm light activate Calcium indicator protein GEM-GECO for neuron image[1]. For the independent excitation of neurons(AWA and AWB) in C. elegans, channelrhodopsins CoChR and Chrimson are activated by blue light and red light[2]. are sensitive to the wavelength of light, it necessary to purify light of the projector.GEM-GECO is activated by 395nm light from LumencorSpectraLumencor LED Illuminator.
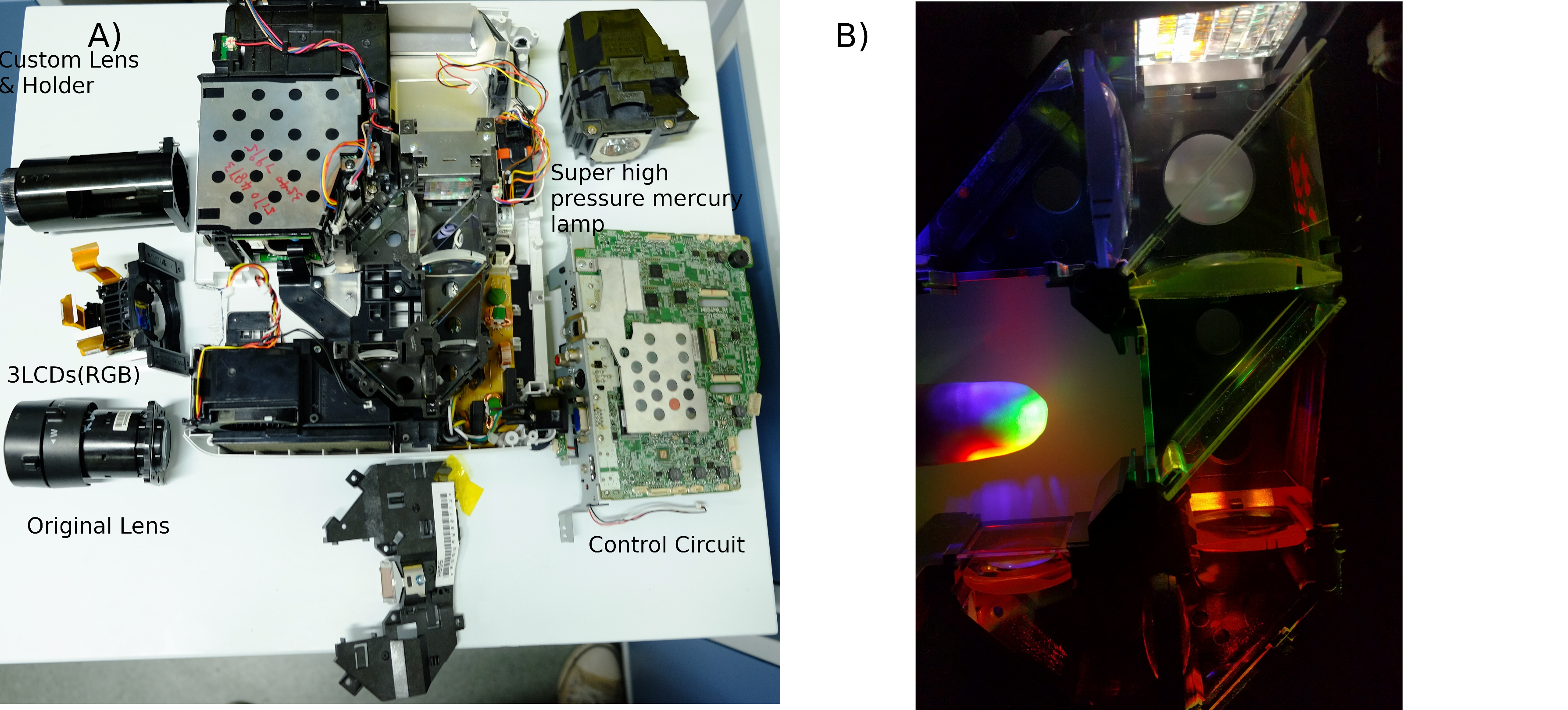
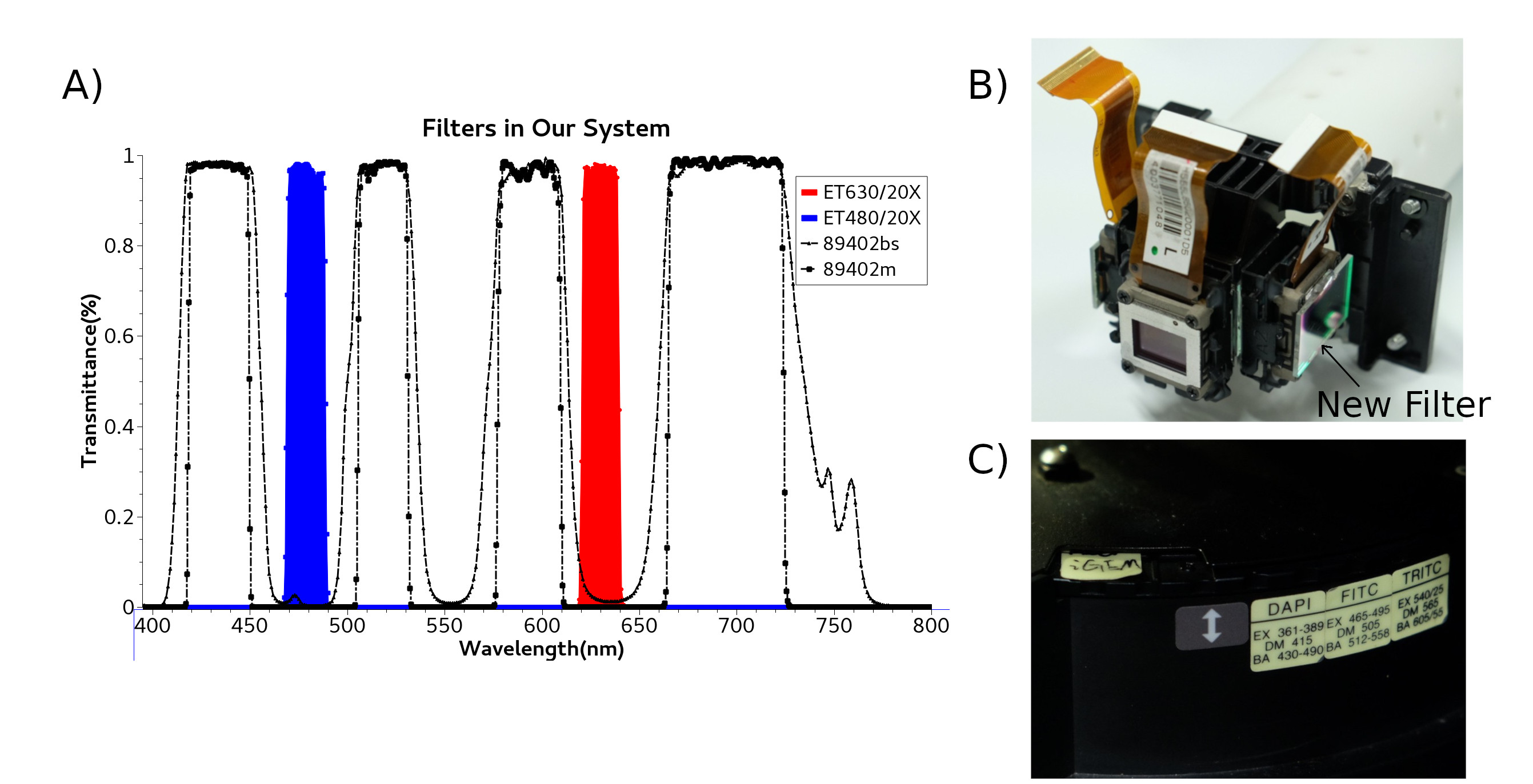
We use projector [http://www.epson.com.cn/products/projectors/239/CB-X03/ EPSON CB-X03] as red light and blue light source. Projector's 3LCDs(red, green, blue channel) function as 1024*768 electronic shutter in each channel. projector's super high pressure mercury lamp as a high-intensity light source. Although original filter can separate white light into red light and blue light, the bandwidth of red light or blue light is not narrow as requirement of our optical sensory protein(CoChR& Chrimson). We install addition red filter Chroma ET630/20X and blue filter Chroma ET480/20X outside 3LCDs.
According to the principle of fluorescence microscope, corresponding Di-Mirror 89402bs and emission filter 89402m are install inside the microscope filter wheel. You can see whole filters we use in our project(Fig).
Mechanical design
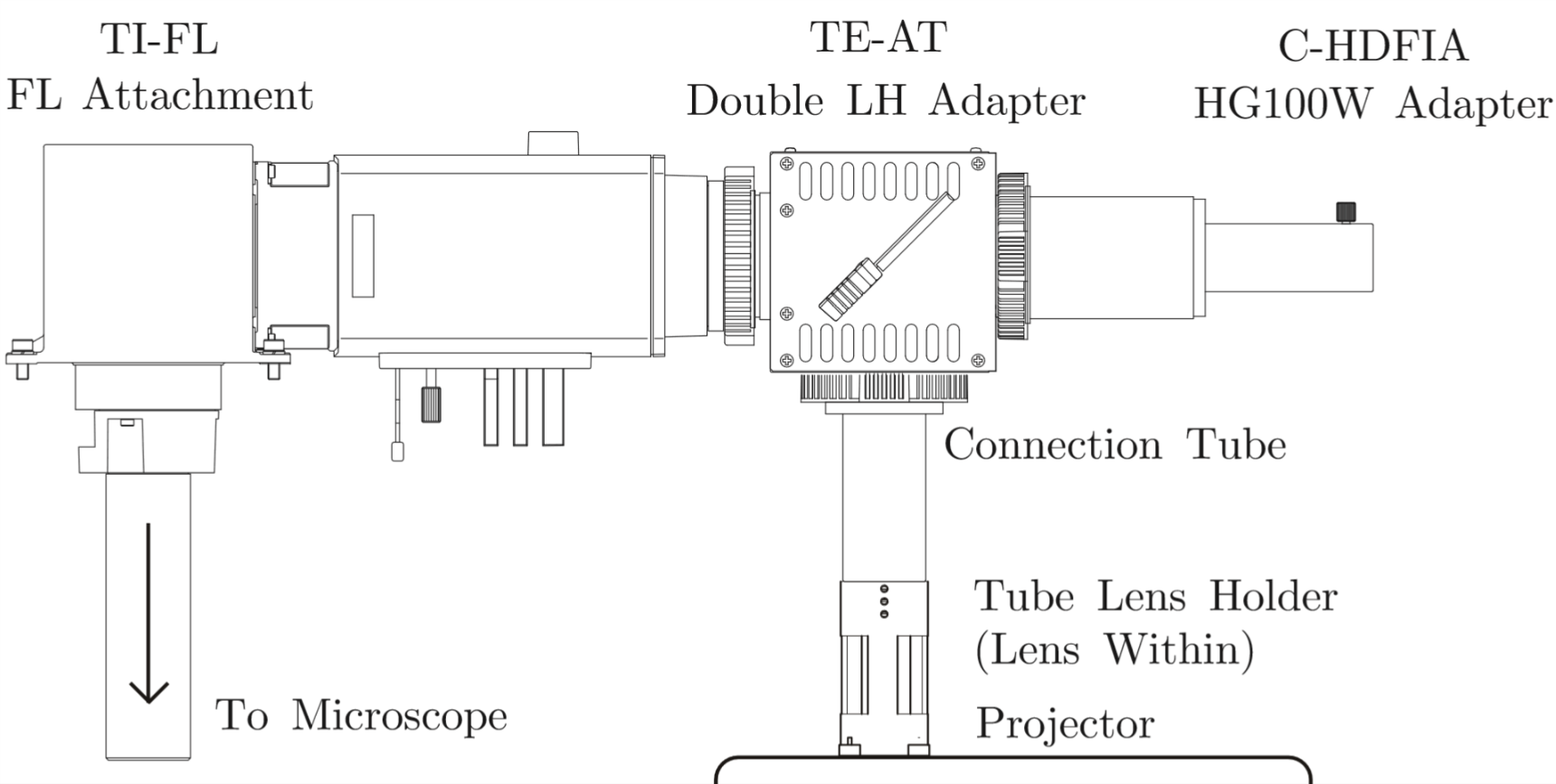
We need to install new lens on projector with adjustable component, and fix all device with microscope stably.
Lens Holder and Connection Tube are design(Solidworks Documents) by CNC and 3D-Print.
Here will be 3D model and real picture.
Control Software
Software is most flexible part. A very easy knack is to use slide(Demo Slide), such as LibreOffice(Test on 5.4.2.2.0+ in Arch Linux) or Microsoft Office. The image and time pattern is stetted in slide, including color, intensity, pulse time etc.
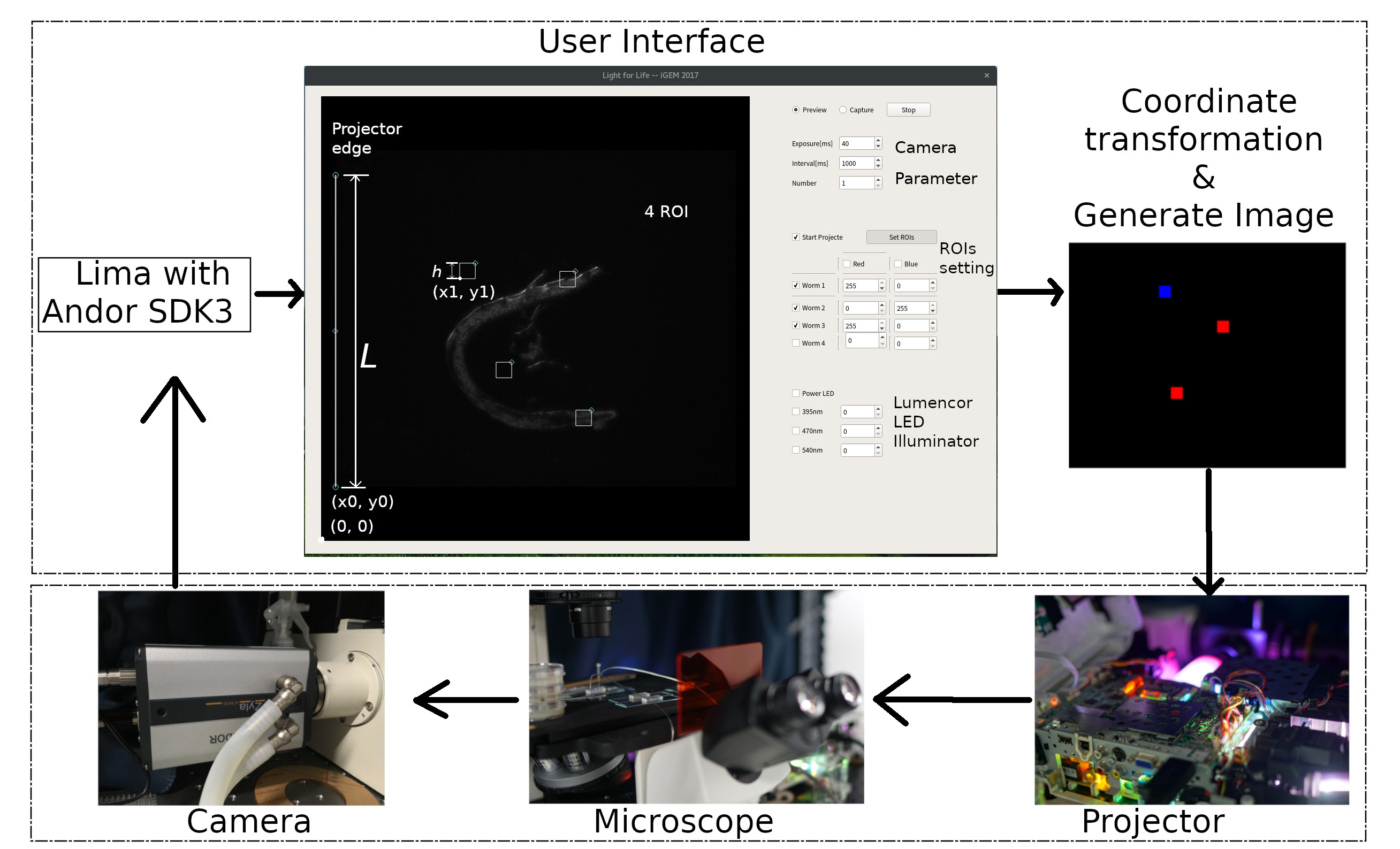
We also develop a more challenged and hackable open source software suit called ColorMapping to track and activate multi C. eleganss or cell independently in one view. User can modify multi color, intensity, time, locations of light alternately in GUI. ColorMapping can be found in GitHub, which still is developing.
ColorMapping, contain camera part, projector part, user&calculation part. It is coded by Python2, Pygraph, PyQt5, [http://lima.blissgarden.org/camera/andor3/doc/index.html?highlight=andor3 Lima(Library for Image Acquisition)]. ColorMapping tests on Arch Linux.
To drive Andor Zyla (5.2), we apply Andor SDK3 from Andor Company firstly. All data are matrix during data transfer and calculation. Camera image live in user interface. In camera part, exposure time and other parameters can be adjusted.
A important algorithm is how to project special area in special color. The key is coordinate system transformation between camera view(2560*2160) and projector(1024*768). To simplify question, only the left projector edge and 4 ROIs are drew. The projector's image will be generated obeying this formula.
\left[\begin{matrix}x_{projector}\\y_{projector}\end{matrix}\right]=[\begin{matrix}{1}&{\frac{1024}{L}}\\ \end{matrix}](\left[\begin{matrix}x_1\\y_1\end{matrix}\right]\left[\begin{matrix}x_0\\y_0\end{matrix}\right])
| Parts | Unit | COST/unit ¥ | COST ¥ |
|---|---|---|---|
| EPSON | 1 | 3960 | 3960 |
| CNC Components | 1 | 600 | 600 |
| Precise 3D-print Component | 1 | 213.22 | 213.22 |
| Optical plate | 1 | 230 | 230 |
| Lifting columns(GCM-2211) | 3 | 210 | 630 |
| Arduino nano v3.0 | 1 | 13.50 | 13.50 |
| Tower-Pro Servo | 1 | 8 | 8 |
| Filter 89402bs | 1 | 3159.45 | 3159.45 |
| Filter ET480/20X | 1 | 2161.73 | 2161.73 |
| Filter ET630/20X | 1 | 2161.73 | 2161.73 |
| Emission Filter 89402 | 1 | 2161.73 | 2161.73 |
| TOTAL COST | 15299.36 |
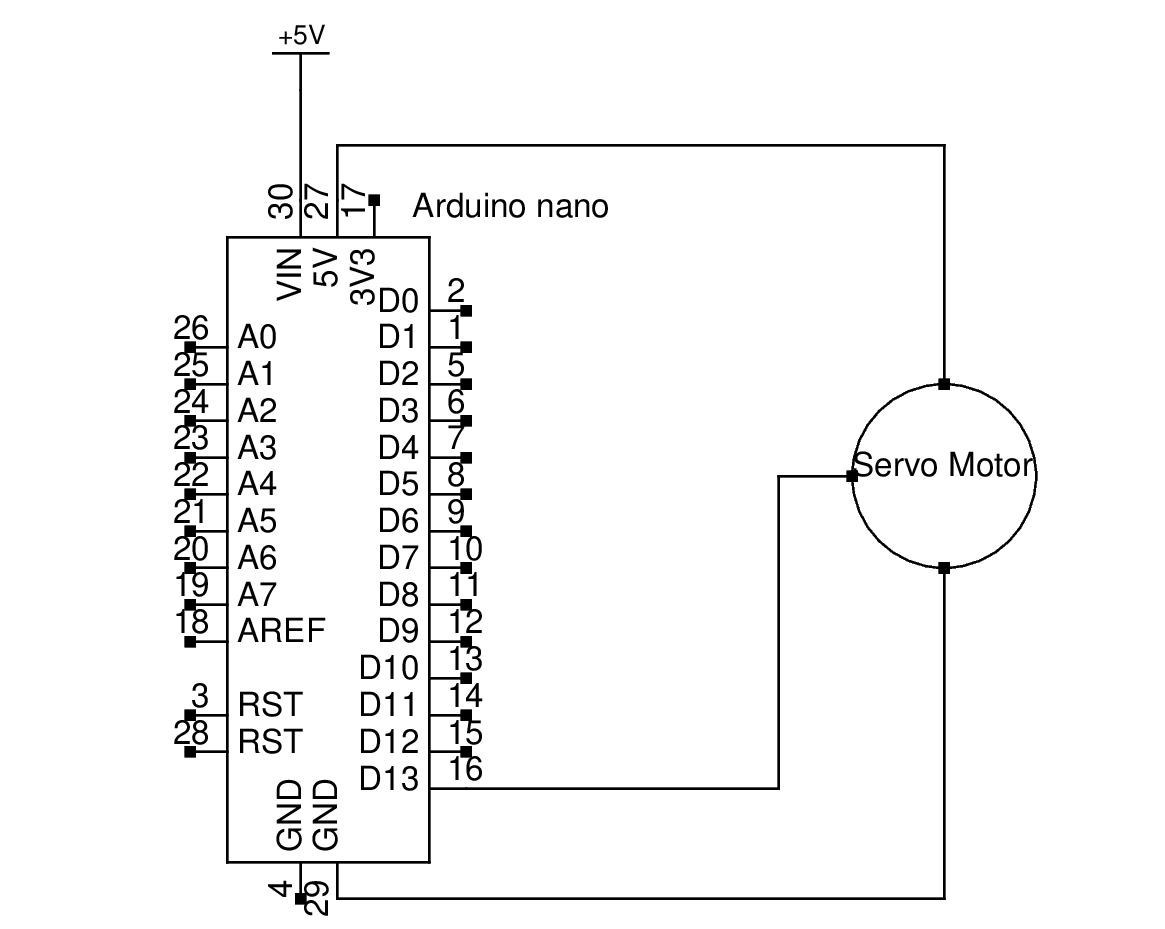
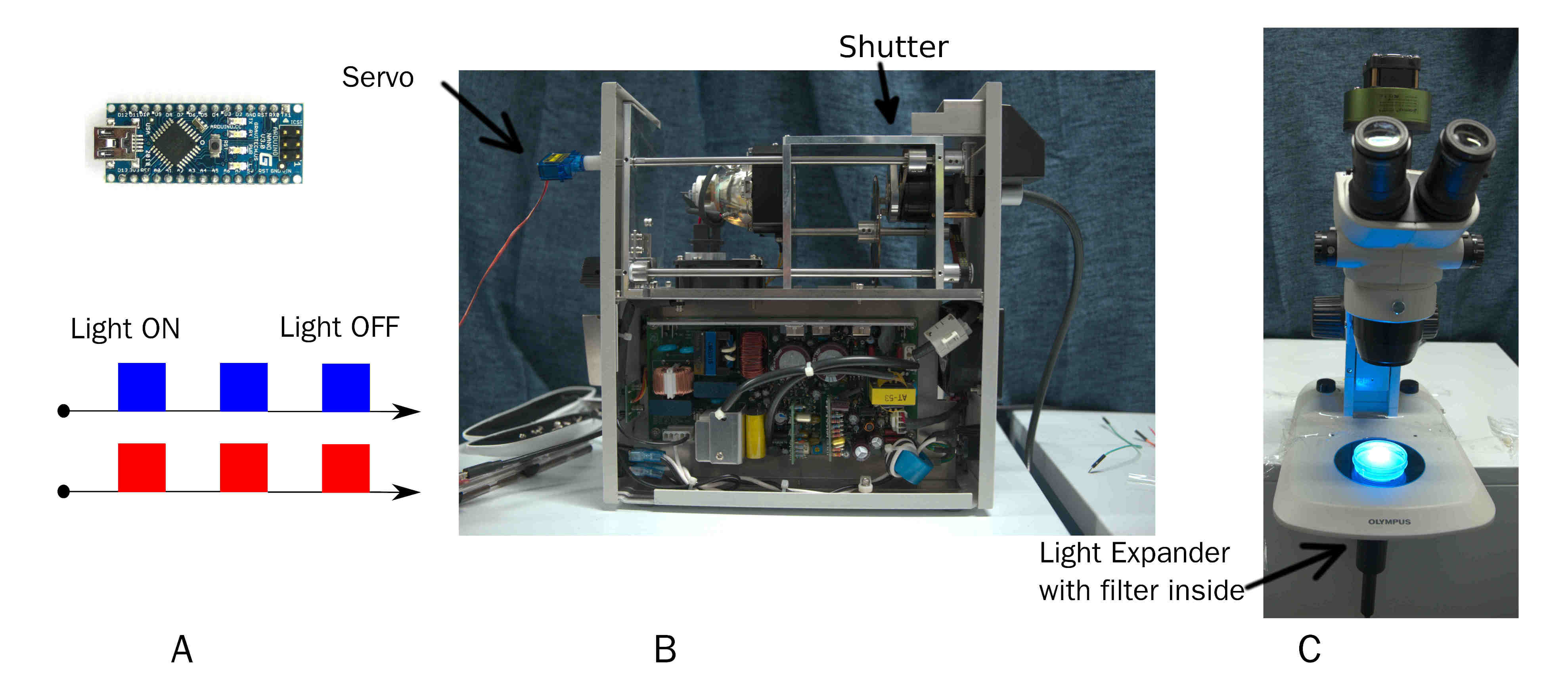
Arduino Modulate Mercury Lamp
Biology background: To reduce the phototoxicity during train C. elegans, pulse of light is required rather than constant light[3].A simple and effective device to output pulse of certain wavelength of light. On time and off time of pulse is custom by Arduino. Wavelength of light is changed by replacing filter before beam expander.
The result can be found in behavior result part.
References
- ↑ Lisa C. Schild and Dominique A. Glauser , Dual Color Neural Activation and Behavior Control with Chrimson and CoChR in Caenorhabditis elegans, 2015, GENETICS.
- ↑ Lisa C. Schild and Dominique A. Glauser , Dual Color Neural Activation and Behavior Control with Chrimson and CoChR in Caenorhabditis elegans, 2015, GENETICS.
- ↑ Venkatachalam, V., & Cohen, A. E. (2014). Imaging GFP-Based Reporters in Neurons with Multiwavelength Optogenetic Control. Biophysical Journal, 107(7), 1554–1563. http://doi.org/10.1016/j.bpj.2014.08.020