Discover our Improved Part
Beta-Lactamase E102F
Engineered highly active Beta-Lactamase mutant E102F
This part is an improvement of BBa_K1189009: Beta-Lactamase with His-Tag.The aim of our project was to generate protein functionality, in vivo through PACE and PREDCEL and in in vitro with help of our software suite AiGEM. To prove that our circuit works, we mutated ß-lactamases based on the scores of our software. Many different mutants were cloned and screened for their minimal inhibitory concentration (MIC). Visit our software validation site here. Typical methods to determine MICs are broth or agar dilutions. The principle is as simple as effective. The microorganism is cultured in liquid medium or on plates with different concentrations of the inhibiting agentIn our case, we want to evaluate differences in the activity of a well characterized protein. Consequently, such an dilution method is ideal. It is easy to setup, very robust and gives exactly the information that is needed for the characterization.
To start the assay, colonies were picked into LB medium with 100 µg/ml chloramphenicol, but without carbenicillin and were grown to the stationary phase for 20 h. Then, deep well plates with 500 µl LB broth per well were prepared. The medium was supplemented with 100 µg/ml chloramphenicol and for each mutant, eight wells with different carbenicillin concentrations, ranging from 10-1280 µg/ml were prepared. Each well was inoculated with 2 µl of the proculture. The new cultures were incubated another 8 h. Finally, the OD600 of each well was measured. By this measure we were able to assert the growth ability of each variant. A first screening revealed, that beside catalytically inactive variants, we also had many candidates that even could survive the highest carbenicillin concentrations (1280 µg/ml)(Fig.: 1). As a result, we decided to perform a second test for these candidates with higher antibiotic concentrations. The three highest carbenicillin concentrations of the first data set were included and the range was extended to a maximum concentration of 19.2 mg/ml. While several candidates turned out to have a weaker activity than the wildtype protein, five of them showed improved properties. The two best candidates could even grow at 19.2 mg/ml (Fig.: 2). The variants were contained one point mutation each, E102F and M67G. These most benefitial mutations, appear in many of our better candidates as well, which underlines there functionality. The outstanding candidate was the E102F mutant, which could even grow at 19.2 mg/ml without affection by the antibiotic. This results in a improvement of at least 100 % in comparison to the wildtype ß-lactamase. This remarkable gain of activity proves, that our software is not only able to recognize protein classes, but also to generate new function.
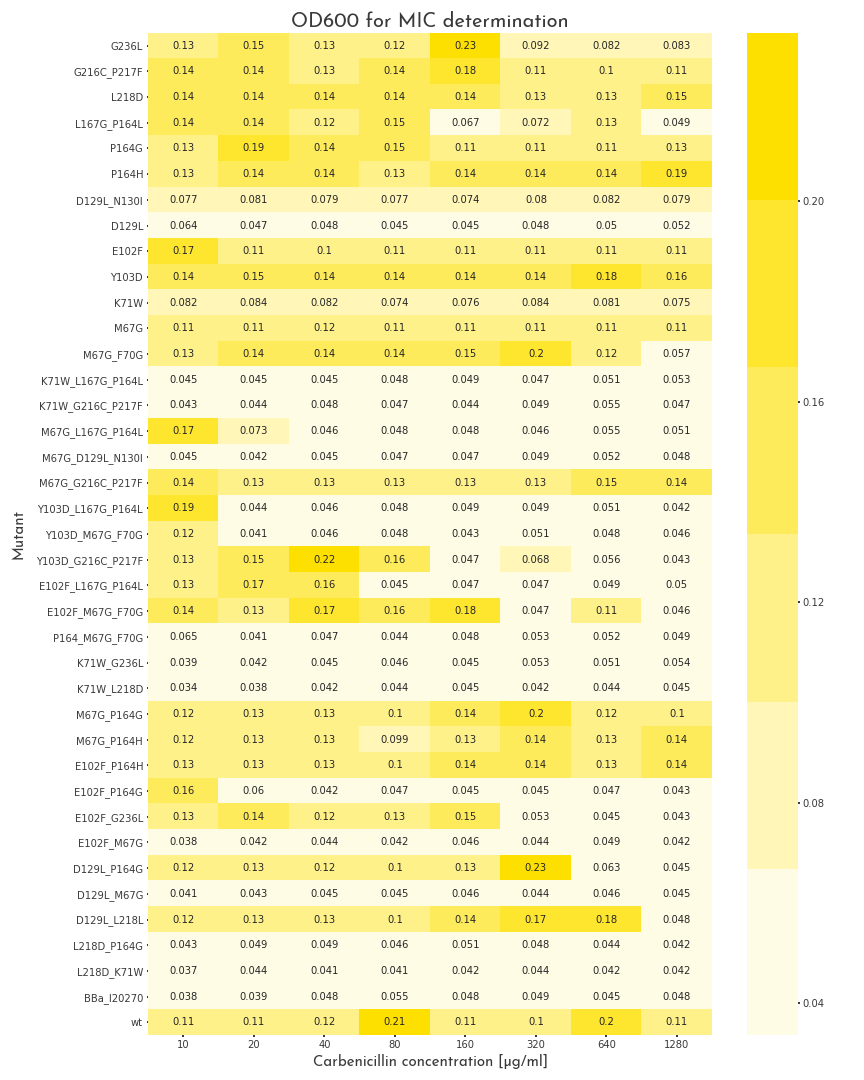
Figure 1: Gowth Behaviour of \(\beta\)-Lactamase Mutants under Different Carbenicillin Concentrations
The plot shows the OD600 of the different \(\beta\)-lactamase mutants at different carbenicillin concentrations. The pool of mutants is very heterogenous. Some proteins are not active at all, some show different grades of activity and 11 mutants, as well as the wildtype enzyme can grow in all conditions.

Figure 2: Gowth Behaviour of \(\beta\)-Lactamase Mutants under Elevated Carbenicillin Concentrations
The plot depicts the \(OD_{600}\) of the \(\beta\)-lactamase mutants that could survive at 1280 \(\frac{µg}{ml}\) in the first assay, under more stringent conditions. The majority of these \(\beta\)-lactamases have a slightly weaker activity than the wildtype, but we could determine five variants with a higher enzymatic activity.

