Joe McKeown (Talk | contribs) |
Joe McKeown (Talk | contribs) |
||
| Line 44: | Line 44: | ||
<section style="padding-top: 10px; padding-bottom: 20px; margin-top: 0px;"> | <section style="padding-top: 10px; padding-bottom: 20px; margin-top: 0px;"> | ||
<div class="container" style="background-color: transparent;"> | <div class="container" style="background-color: transparent;"> | ||
| + | <div class="text-center"> | ||
| + | <h1>Digital Inline Holographic Microscopy</h1> | ||
| + | </div> | ||
<div class="text-left" style="margin: 0px;"> | <div class="text-left" style="margin: 0px;"> | ||
| − | <h2>Part 1: DIHM</h2> | + | <h2>Part 1: Why DIHM?</h2> |
<p style="font-size: 18px;">So, we began the development of an inexpensive DIHM and accompanying software which is able to automatically count cells. Due to the inherently digital nature of this type of microscopy, software can easily be created and adapted such that the DIHM can not only count organisms but, also, differentiate between those that are distinguishable by physical appearance. Among the most important motivators of this hardware/software combination were speed of measurement (our target was the order of seconds or minute per measurement) and low costs for setup and maintenance. Further, in co-cultures wherein neither organism contains a fluorescent marker, the process of counting each type can become a rather complex endeavour. With our QWACC, however, there exists the potential for real-time counting of all physically distinct organisms within a sample.</p> | <p style="font-size: 18px;">So, we began the development of an inexpensive DIHM and accompanying software which is able to automatically count cells. Due to the inherently digital nature of this type of microscopy, software can easily be created and adapted such that the DIHM can not only count organisms but, also, differentiate between those that are distinguishable by physical appearance. Among the most important motivators of this hardware/software combination were speed of measurement (our target was the order of seconds or minute per measurement) and low costs for setup and maintenance. Further, in co-cultures wherein neither organism contains a fluorescent marker, the process of counting each type can become a rather complex endeavour. With our QWACC, however, there exists the potential for real-time counting of all physically distinct organisms within a sample.</p> | ||
<p style="font-size: 18px;">In the table, below, we have compared several methods of cell counting that are able to distinguish between organisms. This is not quantitative and simply shows how each technique stacks up compared to the rest of those on the list. This is denoted through a traffic light system - green indicates that the method is desirable for the given quality, yellow corresponds to a reasonably desirable quality and red indicates that the technique is undesirable with respect to the quality.</p> | <p style="font-size: 18px;">In the table, below, we have compared several methods of cell counting that are able to distinguish between organisms. This is not quantitative and simply shows how each technique stacks up compared to the rest of those on the list. This is denoted through a traffic light system - green indicates that the method is desirable for the given quality, yellow corresponds to a reasonably desirable quality and red indicates that the technique is undesirable with respect to the quality.</p> | ||
| Line 58: | Line 61: | ||
<p style="font-size: 18px;">In the absence of a DIHM, the technique that we would have most likely used is flow cytometry. This would have been suitable for our needs since, in our modification of <em>Chlamydomonas</em> we inserted a fluorescence gene - Venus "YFP" - and <em>E. coli</em> also fluoresces due to mCherry. This would allow us to count the two organisms by exciting with different wavelengths and measuring the responses. This is a relatively accurate procedure and is not overly time consuming. On the other hand, not every organism is modifiable and therefore it is not always possible to discern the numbers of organisms using this technique. For more thorough comparisons between the techniques specified in the above table, | <p style="font-size: 18px;">In the absence of a DIHM, the technique that we would have most likely used is flow cytometry. This would have been suitable for our needs since, in our modification of <em>Chlamydomonas</em> we inserted a fluorescence gene - Venus "YFP" - and <em>E. coli</em> also fluoresces due to mCherry. This would allow us to count the two organisms by exciting with different wavelengths and measuring the responses. This is a relatively accurate procedure and is not overly time consuming. On the other hand, not every organism is modifiable and therefore it is not always possible to discern the numbers of organisms using this technique. For more thorough comparisons between the techniques specified in the above table, | ||
see <a class="btn btn-default btn-xs" href="//2017.igem.org/Team:York/Hardware" role="button" style="color:#222; border-color:#222";">here</a>.</p> | see <a class="btn btn-default btn-xs" href="//2017.igem.org/Team:York/Hardware" role="button" style="color:#222; border-color:#222";">here</a>.</p> | ||
| + | <p style="font-size: 18px;"></p> | ||
<br style="line-height: 10px;"> | <br style="line-height: 10px;"> | ||
</div> | </div> | ||
Revision as of 16:39, 7 September 2017
The Project
Upon being asked to find the number of microorganisms in a given sample, the response from members of our iGEM team has invariably been a long sigh and a plea to the realms of the supernatural.
That most human quality - laziness - alongside flaws in traditional techniques, which we will revisit below, has led to us designing a potential solution in the form of a Digital Inline Holographic Microscope (DIHM).
In particular, we discovered that, when studying co-cultures, there are precious few methods of counting cells that are accurate, fast, cheap and non-destructive.
We decided, therefore, to try to bring together two promising areas of science in this project:
Digital Holographic Microscopy and Co-Culturing.
In the Beginning...
When the York iGEM 2017 team first got together, we knew that co-culturing of microorganisms has become an extremely promising approach, in Biology. In particular, this is true with regards to understanding natural and synthetic cell population interactions and applications in Industrial Biotechnology (e.g. manufacturing and drug research). Our original plan was to use a co-culture comprising Chlamydomonas reinhardtii and Escherichia coli in order to create a somewhat self-sustaining bioreactor that could cost-efficiently produce biofuels using cheap feedstocks. In such an instance, C. reinhardtii would be engineered to export maltose, which E. coli could use in its production of biofuel. Since the crux of this challenge lay in ensuring that C. reinhardtii would export maltose, we planned to use a strain of E. coli that produces ethanol, rather than over-complicate the experiment by using biofuel producing strains that may not have grown as efficiently.
We soon became acutely aware that, along with the maintenance of stable and productive co-cultures being technically challenging, the usual methods of counting cells are often inaccurate, expensive, slow or destructive when applied to cultures of more than one organism. Thence, we have come to consider the lack of a Quicker Way to Analyse Co-Cultures (QWACC) a problem that must be rectified in order to advance the possibilities of research with co-cultures. QWACC is an acronym we will use, henceforth, to refer to the hardware and software that perform the Digital Inline Holographic Microscopy and cell counting in this project. We have not, however, abandoned the idea of producing cheaper biofuels with the C. reinhardtii and E. coli co-culture and it remains a centre piece of this project. It has served as a stark and ever-present reminder that the hardware and software we were developing should be easily used in conjunction with actual experiments involving co-cultures.
So, in the beginning, our project became a bipartite entity. One half of the greater whole was the construction of hardware and software related to using a DIHM to count cells in a co-culture. This would include the microscope itself, software that can automatically count cells and an milli-fluidic chamber to hold samples. The other half was the genetic modification of C. reinhardtii such that it would export maltose in a co-culture with E. coli and the monitoring of the numbers of each organism in a sample with a view to optimising ethanol production.
Digital Inline Holographic Microscopy
Part 1: Why DIHM?
So, we began the development of an inexpensive DIHM and accompanying software which is able to automatically count cells. Due to the inherently digital nature of this type of microscopy, software can easily be created and adapted such that the DIHM can not only count organisms but, also, differentiate between those that are distinguishable by physical appearance. Among the most important motivators of this hardware/software combination were speed of measurement (our target was the order of seconds or minute per measurement) and low costs for setup and maintenance. Further, in co-cultures wherein neither organism contains a fluorescent marker, the process of counting each type can become a rather complex endeavour. With our QWACC, however, there exists the potential for real-time counting of all physically distinct organisms within a sample.
In the table, below, we have compared several methods of cell counting that are able to distinguish between organisms. This is not quantitative and simply shows how each technique stacks up compared to the rest of those on the list. This is denoted through a traffic light system - green indicates that the method is desirable for the given quality, yellow corresponds to a reasonably desirable quality and red indicates that the technique is undesirable with respect to the quality.

- Table 1: A qualitative comparison of organism counting techniques. Green: desirable; yellow: reasonable; red: undesirable.
In the absence of a DIHM, the technique that we would have most likely used is flow cytometry. This would have been suitable for our needs since, in our modification of Chlamydomonas we inserted a fluorescence gene - Venus "YFP" - and E. coli also fluoresces due to mCherry. This would allow us to count the two organisms by exciting with different wavelengths and measuring the responses. This is a relatively accurate procedure and is not overly time consuming. On the other hand, not every organism is modifiable and therefore it is not always possible to discern the numbers of organisms using this technique. For more thorough comparisons between the techniques specified in the above table, see here.
Co-Culture
The second half of the project involves the creation of a co-culture comprising Escherichia coli and Chlamydomonas reinhardtii. Since E. coli and C. reinhardtii are separated in size by an order of magnitude, it is possible to use so-called blob detection algorithms to locate, count and distinguish between the two organisms in holograms formed by a DIHM.
Digital Inline Holographic Microscopy
How does it work?
Digital holographic microscopy makes use of diffraction. This a physical phenomenon wherein objects (or apertures) in the path of a source of waves will create patterns in the waves. These patterns depend on the shape of the objects themselves. Since electromagnetic waves are, somewhat unsurprisingly, waves, it is possible to use the diffraction of light to create patterns that we can see.
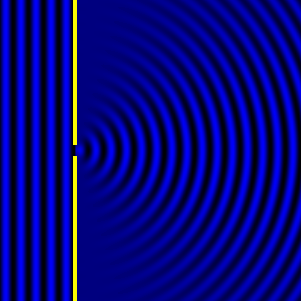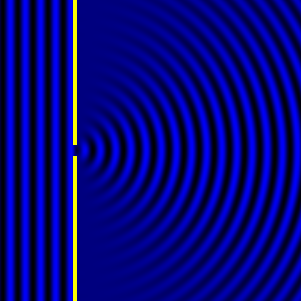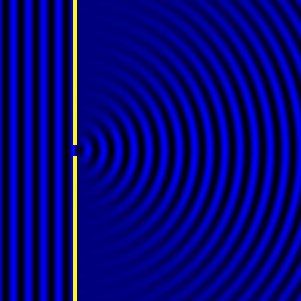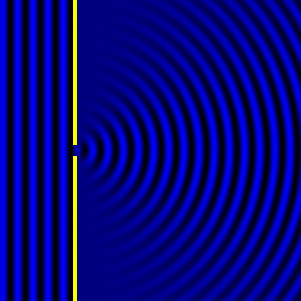
- Diffraction pattern caused by a slit of width equivalent to one wavelength [1].
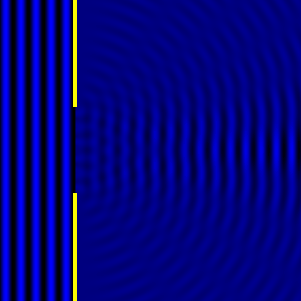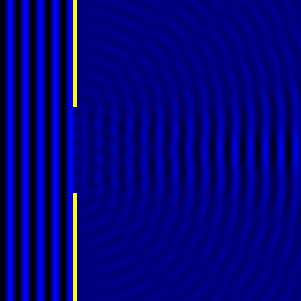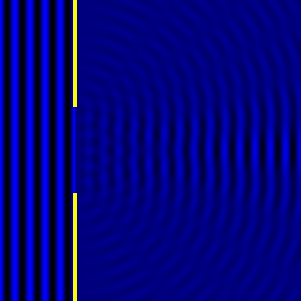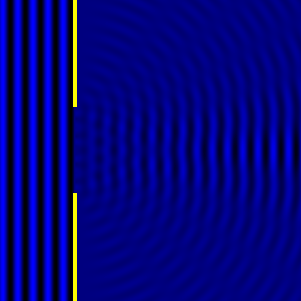
- Diffraction pattern caused by a slit of width equivalent to six wavelengths [1].
The above images show examples of patterns being formed in wavefronts by apertures in the path of otherwise uninterrupted waves. As can be seen by contrasting these images, the pattern created by a larger aperture is distinguishable from a smaller aperture's pattern. The particular distinctions can be described through mathematical formalisms. Thence, once the diffraction patterns have been created, it is possible to infer the shape and size of the objects that caused them, if we also know the wavelength of the light source and the distance from the sample at which the patterns were observed.
References
- [1]: Diffraction Formalism, Wikipedia, as on 4th September 2017 at http://en.wikipedia.org/wiki/Diffraction_formalism



