| (40 intermediate revisions by 7 users not shown) | |||
| Line 3: | Line 3: | ||
{{:Team:SUSTech_Shenzhen/themeCss}}<!--------整体布局,勿动-------------------------------> | {{:Team:SUSTech_Shenzhen/themeCss}}<!--------整体布局,勿动-------------------------------> | ||
| − | {{:Team:SUSTech Shenzhen/templates/page-header|url= | + | {{:Team:SUSTech Shenzhen/templates/page-header-project|url= |
|size=70px|title=Results|subtitle=Project}}<!--------icon在page-header的utl参数里设置,参数size是其大小-------------> | |size=70px|title=Results|subtitle=Project}}<!--------icon在page-header的utl参数里设置,参数size是其大小-------------> | ||
{{:Team:SUSTech_Shenzhen/main-content-begin}}<!---------内容的样式----------------> | {{:Team:SUSTech_Shenzhen/main-content-begin}}<!---------内容的样式----------------> | ||
| Line 10: | Line 10: | ||
| − | == Optical Experiments | + | == Optical Experiments Results == |
| − | + | {|class="table table-striped" | |
| + | |- | ||
| + | | Type of C. elegans | ||
| + | | Fluorescence of mCherry / GFP | ||
| + | | Fluorescence of GEM-GECO | ||
| + | | CoChR work with GEM-GECO | ||
| + | | CoChR work in behavioral Experiments | ||
| + | |- | ||
| + | | Odr10::CoChR::GEM-GECO::mCherry worms | ||
| + | | <html><i class="ion-checkmark"></i></html> mCherry is very bright and beautiful(Fig.1 & 2) | ||
| + | | <html><i class="ion-help"></i></html> weak fluorescence in 497~527nm. Weak change after add diacetyl (Fig. 3). | ||
| + | | <html><i class="ion-load-a"></i></html> Still testing | ||
| + | | <html><i class="ion-checkmark"></i></html> Successful.(See [https://2017.igem.org/Team:SUSTech_Shenzhen/Results#Behavioral_Experiments here]) | ||
| + | |- | ||
| + | | Str1::Chrimson::GEM-GECO::GFP worms | ||
| + | | <html><i class="ion-checkmark"></i></html> GFP were observed in AWB neurons (Fig. 4) | ||
| + | | <html><i class="ion-help"></i></html> weak fluorescence | ||
| + | | <html><i class="ion-load-a"></i></html> Still testing | ||
| + | | <html><i class="ion-loop"></i></html> Exist response, but need more experiment to confirm. | ||
| + | |} | ||
| − | |||
| − | |||
| − | |||
| − | == | + | * mCherry expresses successfully in odr10::CoChR::GEM-GECO::mCherry worm in AWA neurons. The 3D video was captured by [http://luxendo.eu/ Luxendo Light-Sheet Microscope]. |
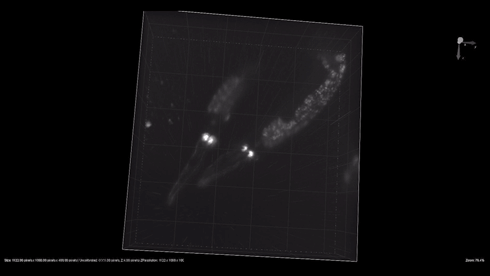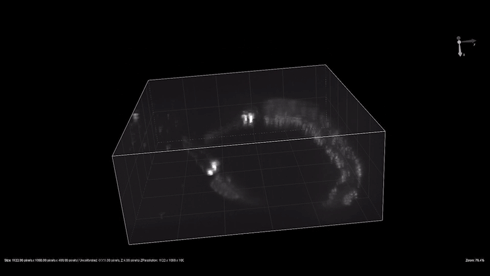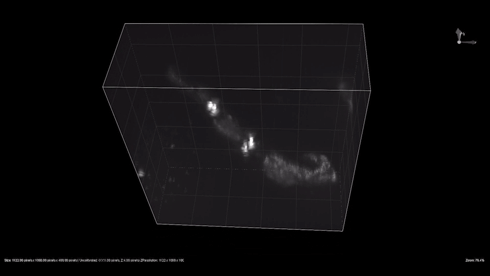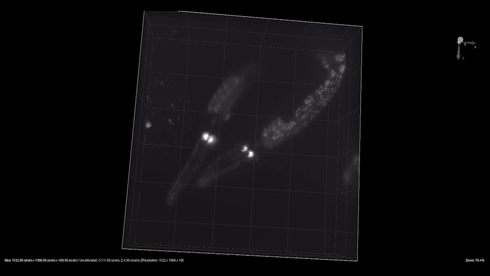
| + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--3dworm.gif|width=800px|caption=Fig. 1 The 3D reconstruction of the odr-10::CoChR::GEM-GECO::mCherry worms' mCherry in AWA neurons. Here are two worm in video. Each worm have two light point, which are pair of AWA neurons.}} | ||
| + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--mCherry.jpg | caption=Fig. 2 Z project of confocal microscope sequences of mCherry in AWA.}} | ||
| − | + | * Emission change at 497~527nm of GEM-GECO after adding diacetyl. It maybe was positive result, but need more control experiments to confirm it. | |
| + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--GEM_GECO.jpg|caption=Fig. 3 Emission change at 497~527nm of GEM-GECO in odr-10::CoChR::GEM-GECO::mCherry after adding diacetyl.}} | ||
| − | == | + | * GFP expresses successfully at AWB in str1::Chrimson::GEM-GECO::GFP worm. |
| + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--C.elegans_AWB_GFP.png | ||
| + | |caption=Fig. 4 GFP expresses in AWB}} | ||
| − | + | == Microfluidic Experiments == | |
| − | + | Here, we fixed the <i>Caenorhabditis elegans</i> in <i>the Immobilization Chip</i> to observe the Odr10::CoChR::GEM-GECO::mCherry worms under the fluorescence microscope and saw the neuronal activity successfully, which can confirm that the worms can express our target genes. We also put the Odr10::CoChR::GEM-GECO::mCherry worms into the chip, after a few minutes the worms would be inactive, then we can "wake up" the worms by the blue light. | |
| − | + | {{SUSTech_Image_Center_fill-width | filename=T--SUSTech_Shenzhen--Microfuildics--result00.png|width=800px|caption=<B>Fig.1 A. <i>The Immobilization Chip</i>. B. The worms in <i>the Immobilization Chip</i>.</B>}} | |
| − | + | Then, we demonstrated that the insertion did not damage the olfactory receptor neuron pairs of the worms by testing their response to diacetyl and 2-nonanone in <i>the Gaussian Plate</i>. | |
| − | + | {{SUSTech_Image_Center_fill-width | filename=T--SUSTech_Shenzhen--Microfuildics--result011.png|width=800px|caption=<B>Fig.2 A. <i>The Gaussian Plate</i>. B. The worms in <i>the Gaussian Plate</i>.</B>}} | |
| − | + | <html><a target="_blank" href="https://2017.igem.org/Team:SUSTech_Shenzhen/Results/Microfluidic" class="btn btn-default"><i class="ion-arrow-right-c"></i> See Details</a></html> | |
| − | == | + | == Behavioral Experiments == |
| − | The | + | Here, we confirmed that the Odr10::CoChR::GEM-GECO::mCherry worms could sense the blue light by inducing the Odr10::CoChR::GEM-GECO::mCherry worms to crawl a cycle on NGM plate. The Odr10::CoChR::GEM-GECO::mCherry worms could follow the blue light spot just like the attract of the food. |
| − | {{ | + | {{SUSTech_Image_Center_fill-width | filename=T--SUSTech_Shenzhen--Microfuildics--result012.png|width=800px|caption=<B>Fig.3 A. The device for light inducing exoeriment made by mercury lamp and optical fiber. B. The worm under the microscope when doing inducing experiment.</B>}} |
| + | Then, in order to study the worms' learning ability we put the worms in alcohol layer on the NGM plate and stimulated them by the blue light at the same time. After 2 hours' training we found that the worms could crawl towards to the alcohol. | ||
| − | + | {{SUSTech_Image_Center_fill-width | filename=T--SUSTech_Shenzhen--Microfuildics--result013.png|width=800px|caption=<B>Fig.4 A.The device made by mercury lamp and microscope for training the worms.(More details in [https://2017.igem.org/Team:SUSTech_Shenzhen/Hardware#Light_Modulator Hardware] B. Using the alcohol to induce the worms.</B>}} | |
| − | + | <html><a target="_blank" href="https://2017.igem.org/Team:SUSTech_Shenzhen/Results/Behavior" class="btn btn-default"><i class="ion-arrow-right-c"></i> See Details</a></html> | |
| − | + | ==Plasmid Construction Results== | |
| − | + | According to the design of plasmid construction, we constructed Odr-10::CoCHR::GEM-geco::mCherry and str-1::Chrimson::GEM-GECO::GFP fusion genes in backbone pCFJ909 successfully. The fusion gene segments were all be sequenced. | |
| − | + | We also amplify B series plasmid in miniMos system for microinjection. | |
| + | We integrated Odr-10::CoChR::GEM-GECO::mCherry and str-1::Chrimson::GEM-GECO::GFP fusion genes into <i>C. elegans(Caenorhabditis elegans)</i> by microinjection respectively. | ||
| − | + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--Results-plasmid1.png|width=800px|caption=<B>Fig.5 Odr-10::CoCHR::GEM-geco::mCherry</B>}} | |
| − | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen-- | + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--Results-plasmid2.png|width=800px|caption=<B>Fig.6 str-1::Chrimson::GEM-GECO::GFP</B>}} |
| − | + | ==Microinjection Results== | |
| − | + | In order to get <i>C. elegans</i> strains with the preference to blue lights and the aversion to red lights, we used miniMos injection to inject our plasmids in to worms for expression. | |
| − | + | On July.7, we microinjected 20 worms with Odr-10::CoChR::GEM-GECO::mCherry and 20 worms with Str-1::Chrimson::GEM-GECO::GFP. After 3 days, for each kinds of worms, we obtained more than 6 free-moving F1 with fluorescences. Then, 10 days after microinjection, we did heat shock to screen stable inheritance worms. For worms with Odr-10::CoChR::GEM-GECO::mCherry, one plate successfully survived more than 30 worms without GFP(a selective marker), but none of worms with Str-1::Chrimson::GEM-GECO::GFP survived. In addition, we did mapping experiments and demonstrated that Odr-10::CoChR::GEM-GECO::mCherry had successfully inserted in chromosome 1. | |
| − | + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--Results-MI1.png|width=800px|caption=<B>Fig.7 heat shock</B>}} | |
| + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--Results-MI2.png|width=800px|caption=<B>Fig.8 heatshock under fluorenscence microscope</B>}} | ||
| + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--Results-MI3.png|width=800px|caption=<B>Fig.9 CoChR under confocal microscope</B>}} | ||
| − | + | On August 1st, we microinject worms using Str-1::Chrimson::GEM-GECO::GFP. This time we injected 20 worms and also observed F1 phenotype after 3 days, picking up free-moving worms with RFP and did heat shock 9 days later. After heat shock, on August 21th, we got about 10 worms expressed plasmids without arrays, meaning that we obtained stable inheritance worms expressing Str-1::Chrimson::GEM-GECO::GFP. | |
| − | = | + | {{SUSTech_Image_Center_8 | filename=T--SUSTech_Shenzhen--Results-MI4.png|width=800px|caption=<B>Fig.10 Chrimoson under confocal microscope</B>}} |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
{{:Team:SUSTech_Shenzhen/main-content-end}}<!---------内容的样式-----------------> | {{:Team:SUSTech_Shenzhen/main-content-end}}<!---------内容的样式-----------------> | ||
{{:Team:SUSTech_Shenzhen/wiki-footer}} | {{:Team:SUSTech_Shenzhen/wiki-footer}} | ||
{{:Team:SUSTech_Shenzhen/themeJs}}<!--------布局居中-------------> | {{:Team:SUSTech_Shenzhen/themeJs}}<!--------布局居中-------------> | ||
Latest revision as of 03:50, 2 November 2017
Results
Project
Contents
Optical Experiments Results
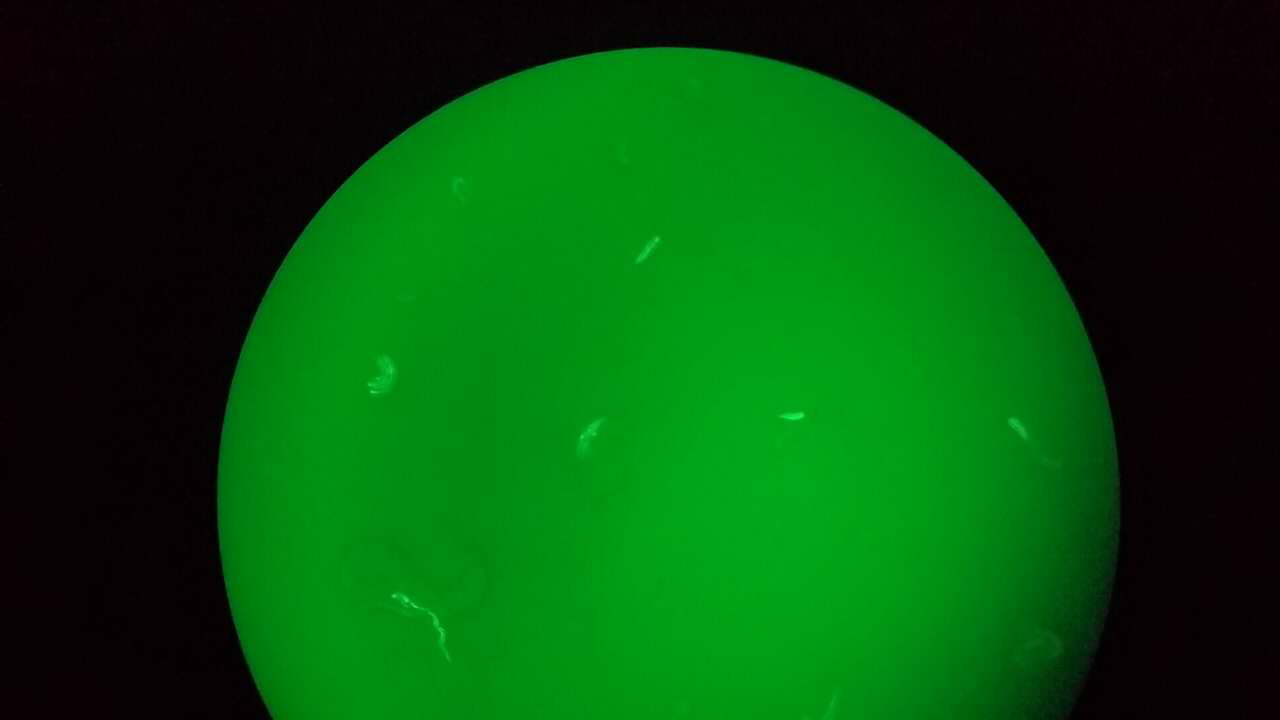
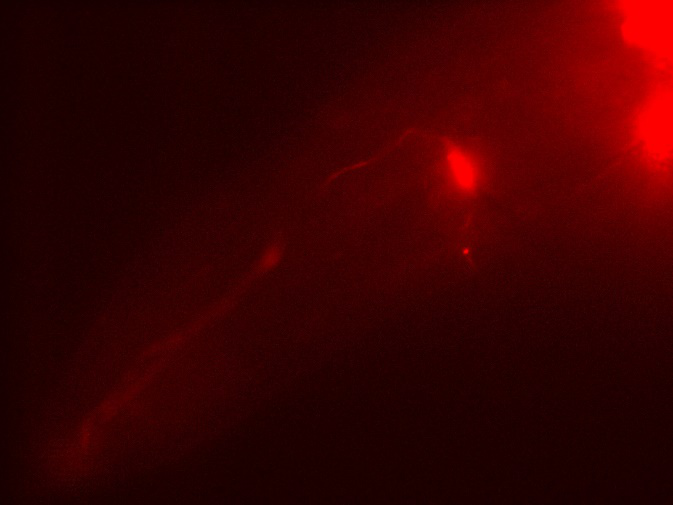
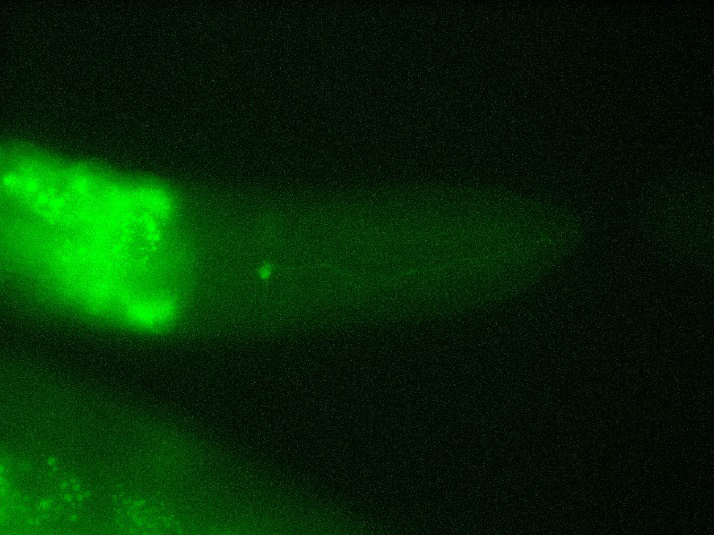
| Type of C. elegans | Fluorescence of mCherry / GFP | Fluorescence of GEM-GECO | CoChR work with GEM-GECO | CoChR work in behavioral Experiments |
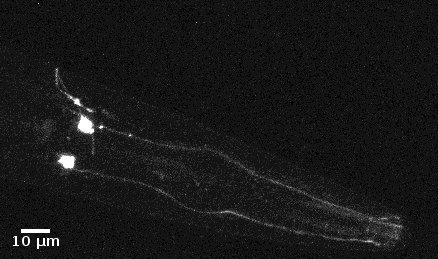
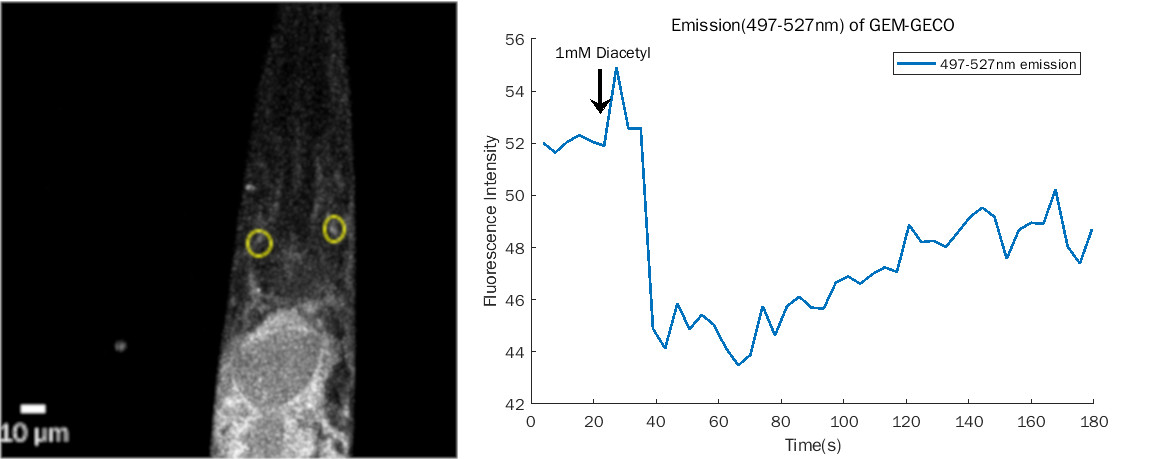
| Odr10::CoChR::GEM-GECO::mCherry worms | mCherry is very bright and beautiful(Fig.1 & 2) | weak fluorescence in 497~527nm. Weak change after add diacetyl (Fig. 3). | Still testing | Successful.(See here) |
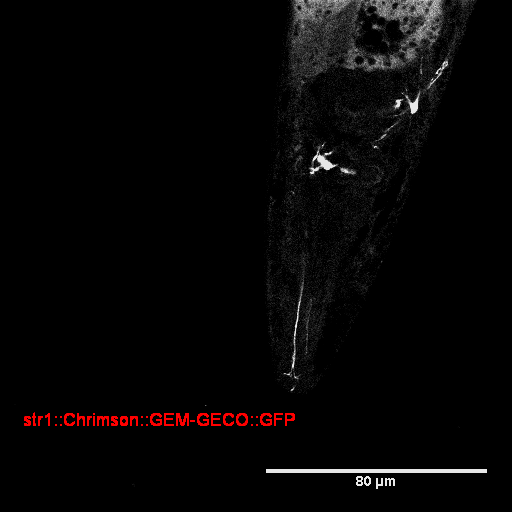
| Str1::Chrimson::GEM-GECO::GFP worms | GFP were observed in AWB neurons (Fig. 4) | weak fluorescence | Still testing | Exist response, but need more experiment to confirm. |
- mCherry expresses successfully in odr10::CoChR::GEM-GECO::mCherry worm in AWA neurons. The 3D video was captured by [http://luxendo.eu/ Luxendo Light-Sheet Microscope].
- Emission change at 497~527nm of GEM-GECO after adding diacetyl. It maybe was positive result, but need more control experiments to confirm it.
- GFP expresses successfully at AWB in str1::Chrimson::GEM-GECO::GFP worm.
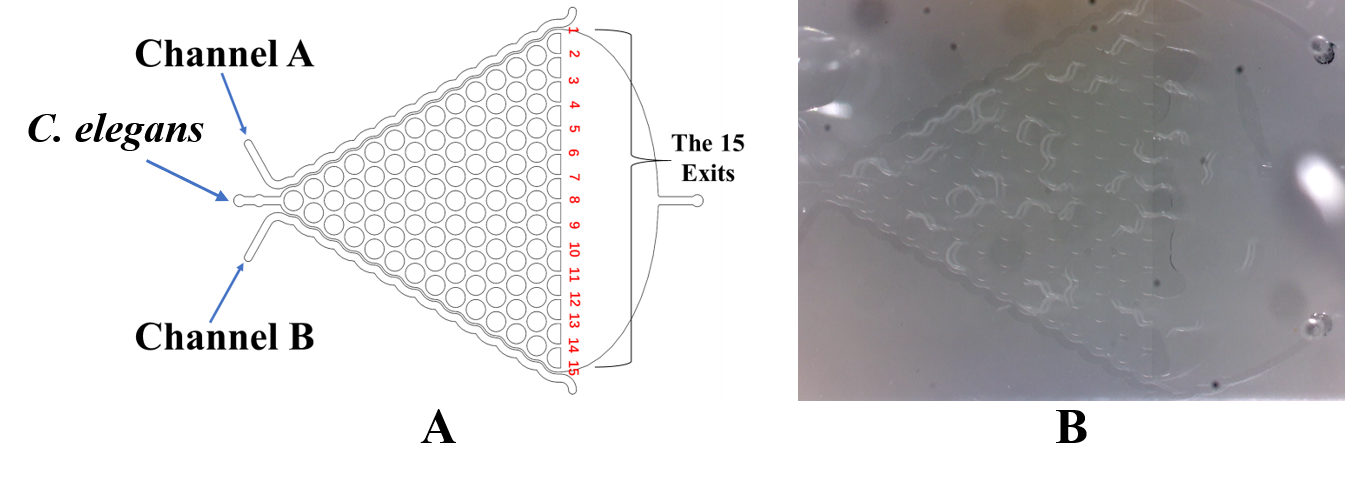
Microfluidic Experiments
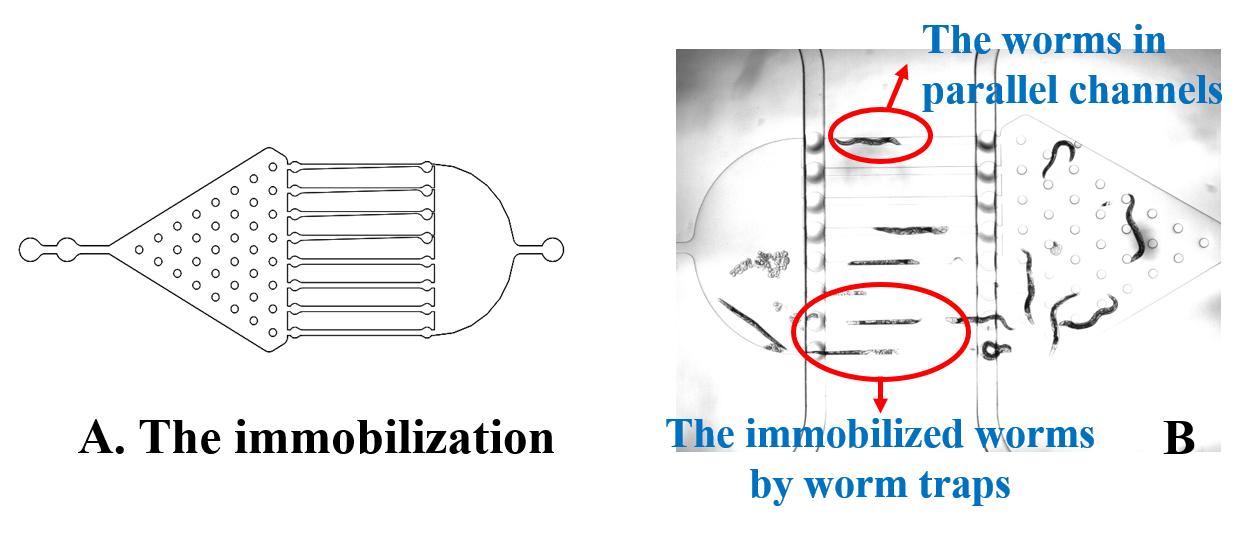
Here, we fixed the Caenorhabditis elegans in the Immobilization Chip to observe the Odr10::CoChR::GEM-GECO::mCherry worms under the fluorescence microscope and saw the neuronal activity successfully, which can confirm that the worms can express our target genes. We also put the Odr10::CoChR::GEM-GECO::mCherry worms into the chip, after a few minutes the worms would be inactive, then we can "wake up" the worms by the blue light.
Then, we demonstrated that the insertion did not damage the olfactory receptor neuron pairs of the worms by testing their response to diacetyl and 2-nonanone in the Gaussian Plate.
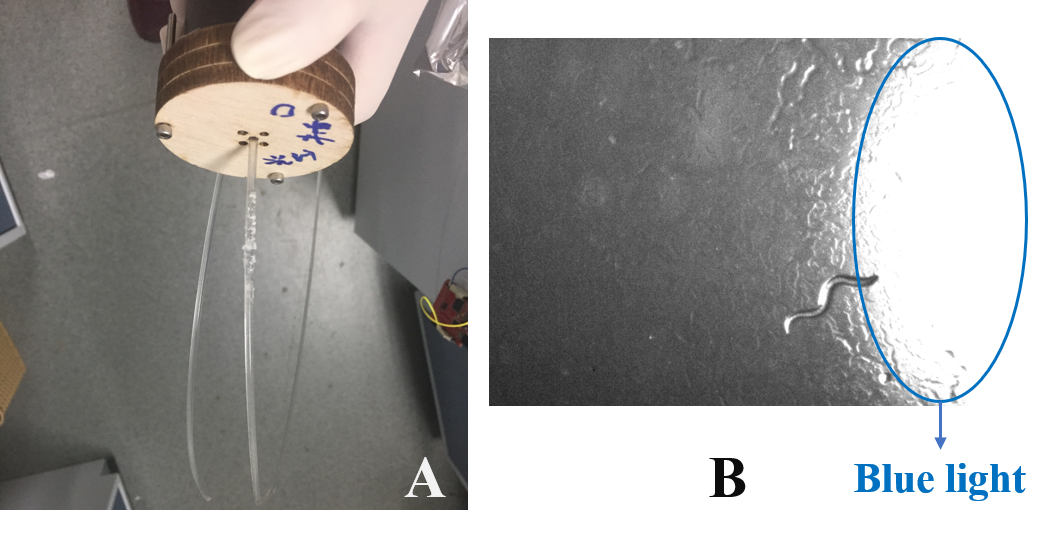
Behavioral Experiments
Here, we confirmed that the Odr10::CoChR::GEM-GECO::mCherry worms could sense the blue light by inducing the Odr10::CoChR::GEM-GECO::mCherry worms to crawl a cycle on NGM plate. The Odr10::CoChR::GEM-GECO::mCherry worms could follow the blue light spot just like the attract of the food.
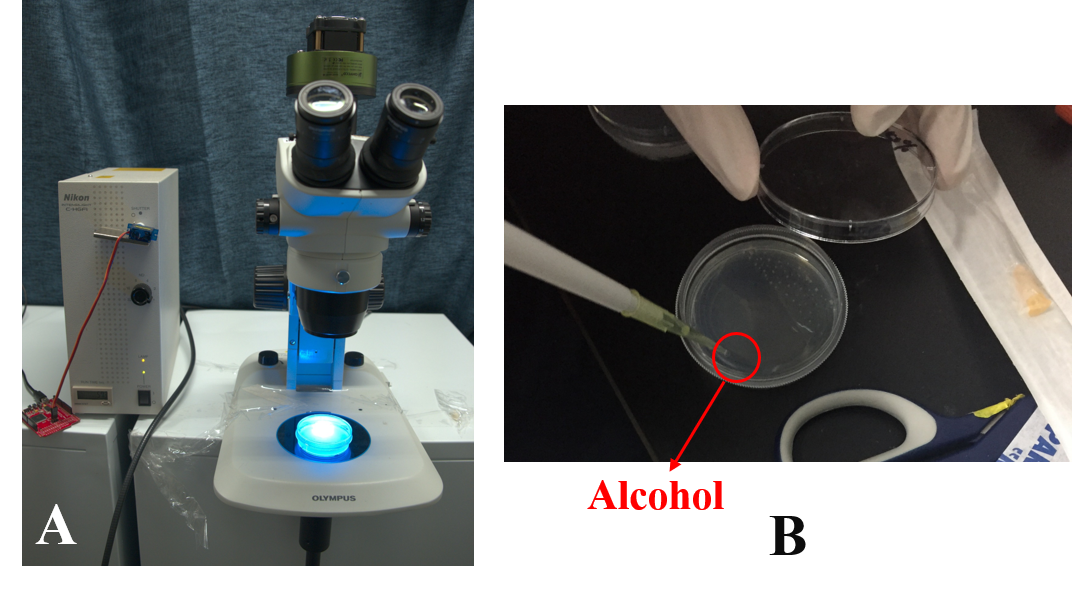
Then, in order to study the worms' learning ability we put the worms in alcohol layer on the NGM plate and stimulated them by the blue light at the same time. After 2 hours' training we found that the worms could crawl towards to the alcohol.
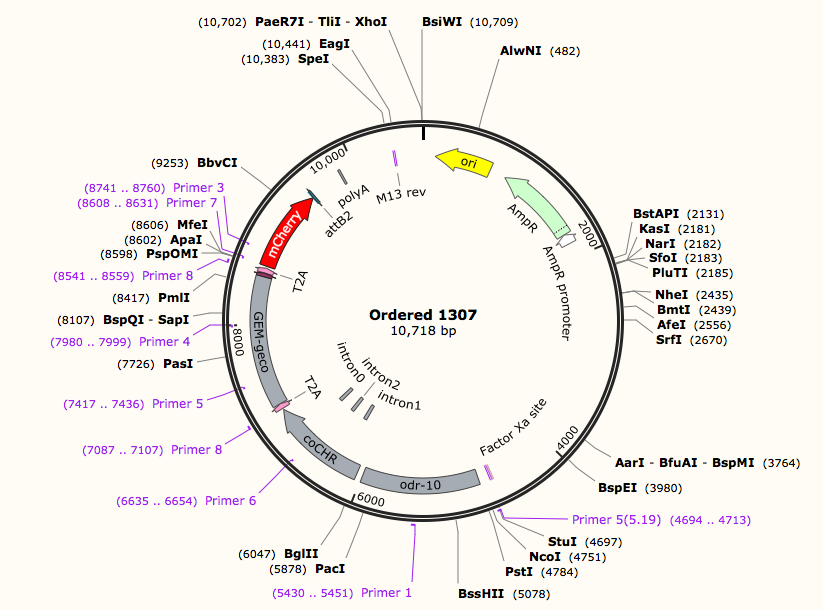
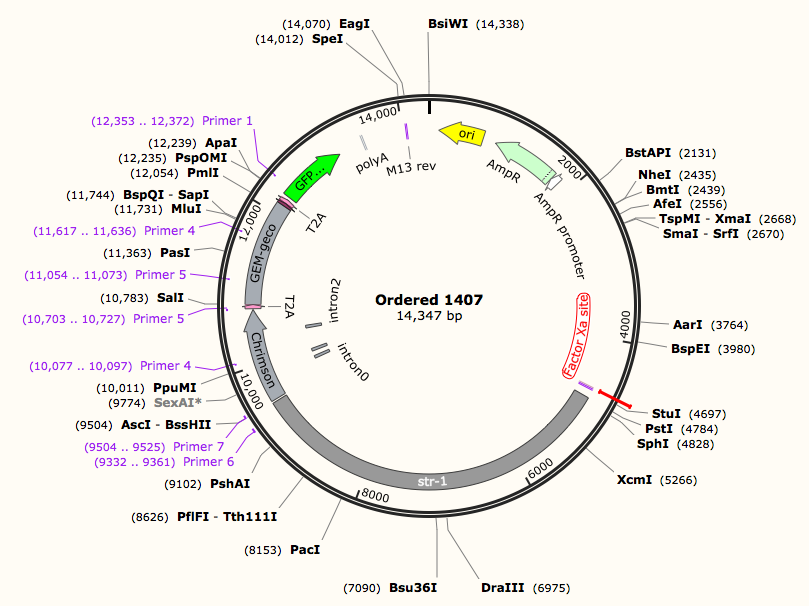
Plasmid Construction Results
According to the design of plasmid construction, we constructed Odr-10::CoCHR::GEM-geco::mCherry and str-1::Chrimson::GEM-GECO::GFP fusion genes in backbone pCFJ909 successfully. The fusion gene segments were all be sequenced.
We also amplify B series plasmid in miniMos system for microinjection. We integrated Odr-10::CoChR::GEM-GECO::mCherry and str-1::Chrimson::GEM-GECO::GFP fusion genes into C. elegans(Caenorhabditis elegans) by microinjection respectively.
Microinjection Results
In order to get C. elegans strains with the preference to blue lights and the aversion to red lights, we used miniMos injection to inject our plasmids in to worms for expression.
On July.7, we microinjected 20 worms with Odr-10::CoChR::GEM-GECO::mCherry and 20 worms with Str-1::Chrimson::GEM-GECO::GFP. After 3 days, for each kinds of worms, we obtained more than 6 free-moving F1 with fluorescences. Then, 10 days after microinjection, we did heat shock to screen stable inheritance worms. For worms with Odr-10::CoChR::GEM-GECO::mCherry, one plate successfully survived more than 30 worms without GFP(a selective marker), but none of worms with Str-1::Chrimson::GEM-GECO::GFP survived. In addition, we did mapping experiments and demonstrated that Odr-10::CoChR::GEM-GECO::mCherry had successfully inserted in chromosome 1.
On August 1st, we microinject worms using Str-1::Chrimson::GEM-GECO::GFP. This time we injected 20 worms and also observed F1 phenotype after 3 days, picking up free-moving worms with RFP and did heat shock 9 days later. After heat shock, on August 21th, we got about 10 worms expressed plasmids without arrays, meaning that we obtained stable inheritance worms expressing Str-1::Chrimson::GEM-GECO::GFP.