| Line 26: | Line 26: | ||
The second plan is making the grows of worms synchronously, which is utilized to get a large number of worms at the same stage (链接到微流控protocol). After collecting embryos (Fig. 3) by bleaching adults, we culture them and get a large number of worms at the same stages after three days. our synchronous rate is calculated as the formula below. | The second plan is making the grows of worms synchronously, which is utilized to get a large number of worms at the same stage (链接到微流控protocol). After collecting embryos (Fig. 3) by bleaching adults, we culture them and get a large number of worms at the same stages after three days. our synchronous rate is calculated as the formula below. | ||
| − | {{SUSTech_Shenzhen/bmath|equ=<nowiki>\frac{N_1*100}{N_2}</nowiki>}} | + | {{SUSTech_Shenzhen/bmath|equ=<nowiki>\frac{N_1*100\%}{N_2}</nowiki>}} |
<html><p class="text-center">N<sub>1</sub> equals the number of worms at L4</p> <p class="text-center">N<sub>2</sub> equals the number of all worms.</p></html> | <html><p class="text-center">N<sub>1</sub> equals the number of worms at L4</p> <p class="text-center">N<sub>2</sub> equals the number of all worms.</p></html> | ||
Revision as of 17:38, 31 October 2017
Microfluidics
Create for wisdom of Life
Contents
Microfluidics is both a science and a technology that is currently an active field of academic research and study. It consists of systems that work with small volumes of fluids in the nanoliter/microliter scale, through channels ranging from tens to hundreds of micrometers in diameter. It is well matched with the size of Caenorhabditis elegans. In this system, we can study a group of worms as well as an individual one clearly, efficiently and conveniently.
In order to test whether insertion affect C. elegans's previous function in population, we need to get their freely response. In addition, we want to test worms individually by activing the receptors on neurons. The semifixed is necessary in this test. In order to get an efficient experimental results, we need synchronous worms. Because of these demands above, we design three microfluidics: the selection chip, the Gaussian Plate, and the Immobilization chip. (Fig. 1)
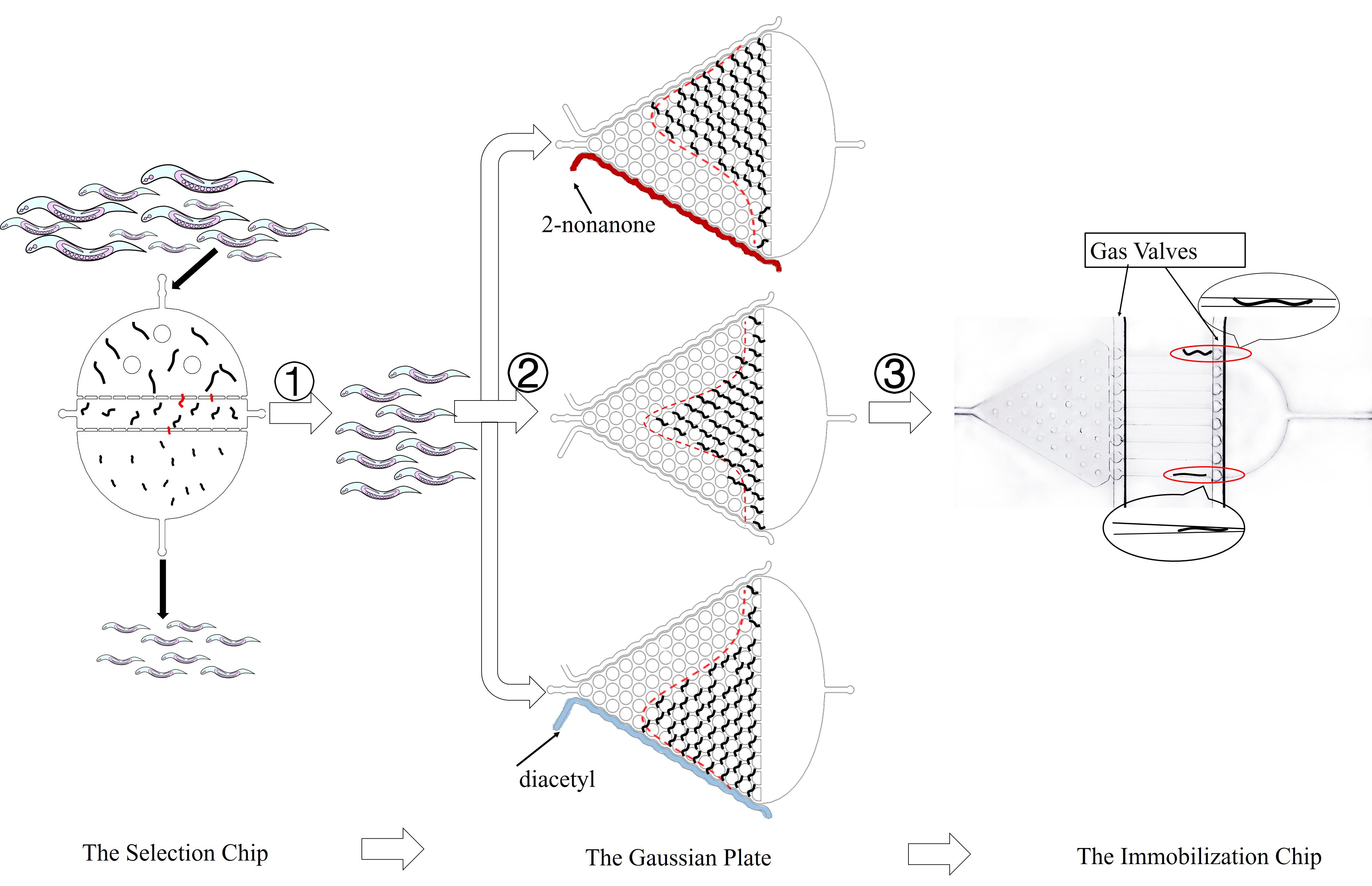
The Design of Microfluidics
1 The Selection Chip
Most researches about C. elegans show that inserted genes can express well in worms at L4 stages. Thus, we need to select appropriate stages of worms to get the best experimental results. The simple method is to distinguish them by sizes, because worms in L4 stage have medium sizes. There are two plans of selecting worms. The first one is using microfluidics. With the flow contained worms going through this chip, only the medium sized worms can remain in the medium chamber, and we could collect them by injecting the flow from the bottom and gather them in the top. (Fig. 2)
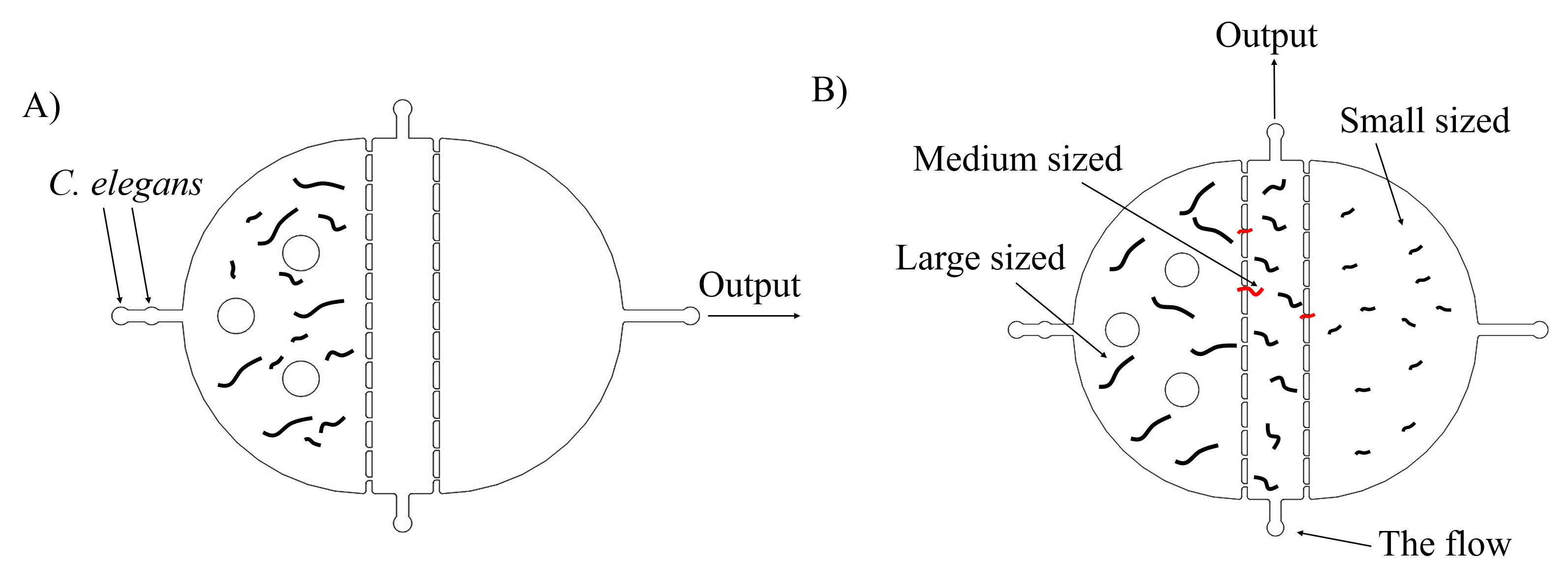
The second plan is making the grows of worms synchronously, which is utilized to get a large number of worms at the same stage (链接到微流控protocol). After collecting embryos (Fig. 3) by bleaching adults, we culture them and get a large number of worms at the same stages after three days. our synchronous rate is calculated as the formula below.
\frac{N_1*100\%}{N_2}
N1 equals the number of worms at L4
N2 equals the number of all worms.
The successful rate can reach to about 80%.
Compared with those two methods in experiment, we find that we can get almost 150 of 100 worms (One adult have no less than 3 embryos) in three days by the synchronization method, while we can just get 20 of 100 worms in one day by microfluidics method. Given that we need a large number of worms to do the following experiment, we think the synchronization method is better. You can get more detail results by clicking See Details.
2 The Gaussian Plate
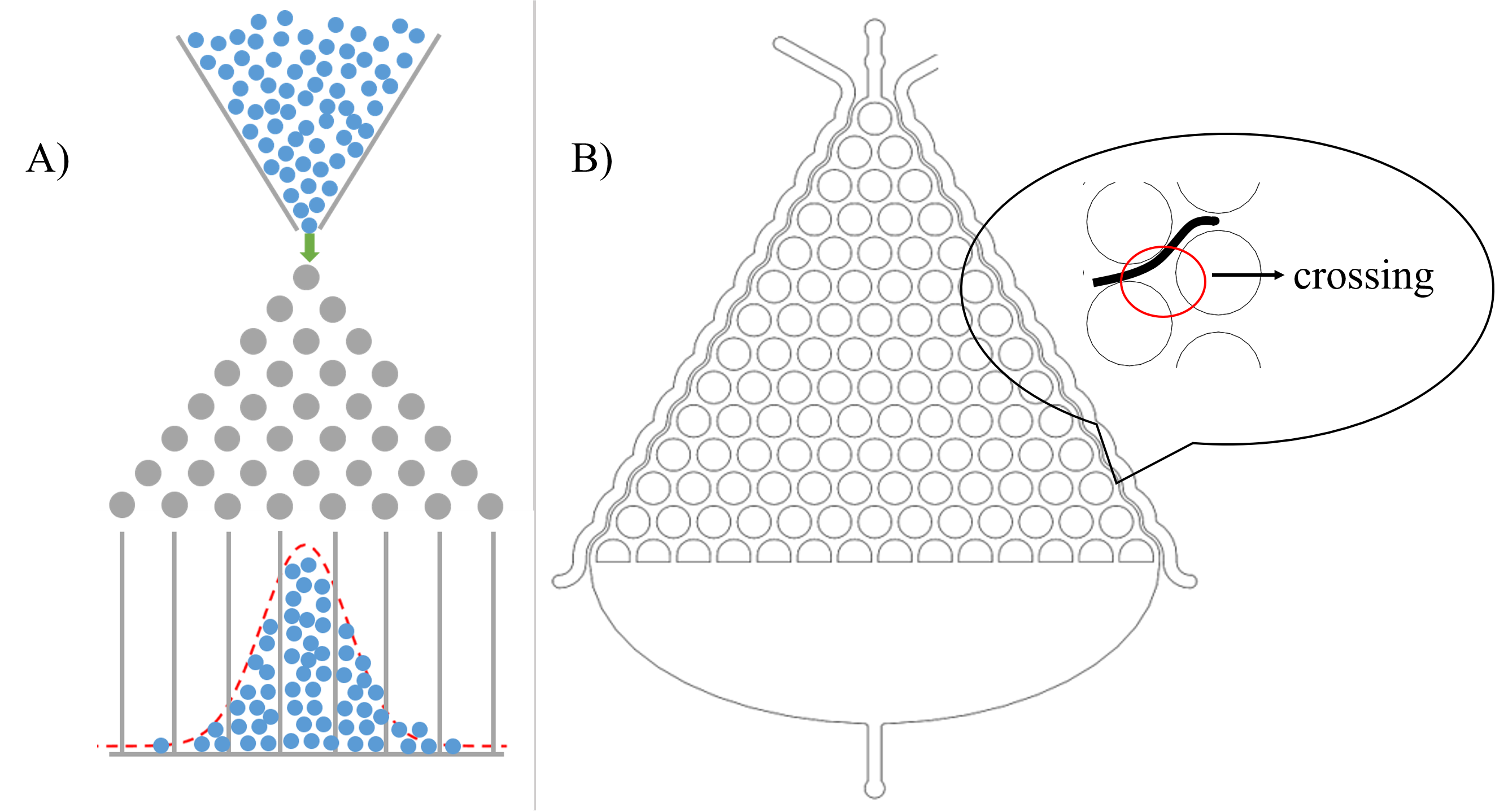
In order to study locomotive behavior of C. elegans populations, we design the Gaussian Plate, a pillar-filled area, where the pillars are designed such that it allows crawling-like behaviors even though worms are immersed in liquid environment.(Fig. 4) [1].
After deciding to use the microfluidics to study the locomotive behavior, we are noticed that the shape of microfluidics is similar to the Galton board.[2]. (Fig. 5(A)) Therefore, we assume that the probability for C. elegans choose to go left or right is equal when it passes a crossing. (Fig. 5(B))
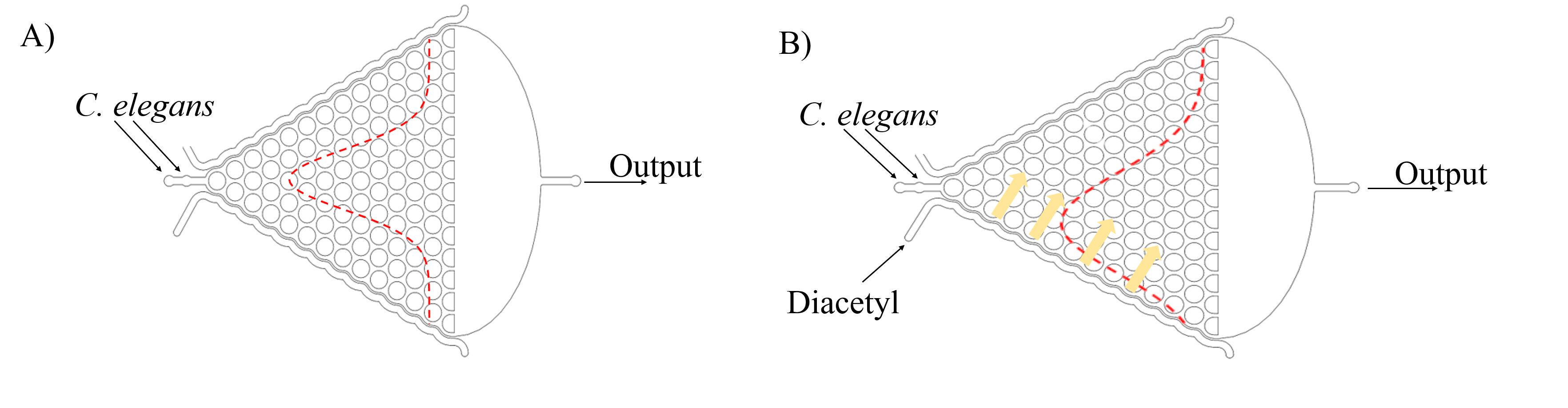
In addition, we can assume that C. elegans is just like balls in the Galton board. The force of slow buffer flow acting on worms is the same as the gravity acting on balls. Both of the distribution is Gaussian distribution. Given that we need to make sure that injecting the target genes in C. elegans will not affect its olfactory receptor neuron pair, we injected diacetyl (2-nonanone) that C. elegans prefers (repulse) into the right (left) channel to make a concentration gradient on Gaussian Plate. Because of the gradient, worms tend to move to the side filled with diacetyl, causing Gaussian distribution changed. (Fig. 6)
In order to make a concentration gradient, we come up with two methods to get it. (Fig. 7)
In order to simulate the process of diffusion, we make a diffusion model to guide us. More details. See Details.
Both of those methods could be carried out theoretically. But in the process of experiment, we find the method 1 (Inject chemicals into the side of layer 1) is better.
3 The Immobilization Chip
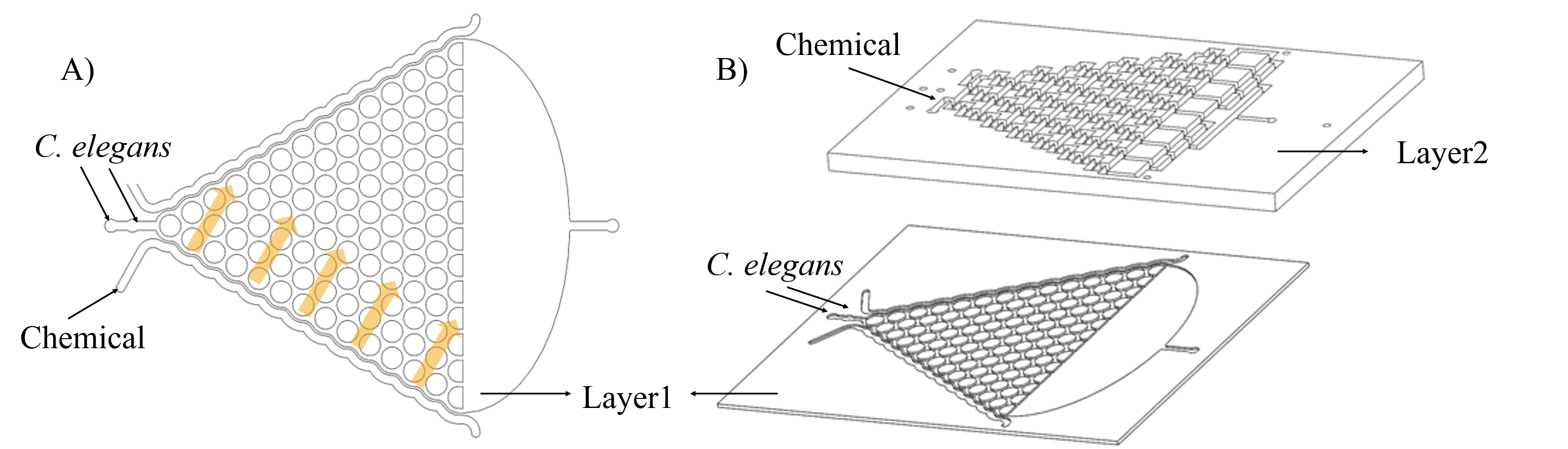
After studying worms’ group behaviors and proving their olfactory neurons are not affected by exogenous gene, we could study their individual neuron activity and behavioral response under a light stimulus of specific wavelength. Traditionally, anesthetics and glues are utilized to immobilize worms. However, worms will be damaged in this condition and it will make it difficult to study the behavioral response of worms. Thus, we designed two kinds of microfluidic chips to allow high-resolution microscopic imaging on chip without damaging for worms. (Fig. 8)
The first kind of channel is trapping worms in the wedge-shaped channel, called worm clamps. It is utilized to study the contraction and elongation of their heads and research the neuronal activity by detecting calcium indicator GEM-GECO in imaging software.
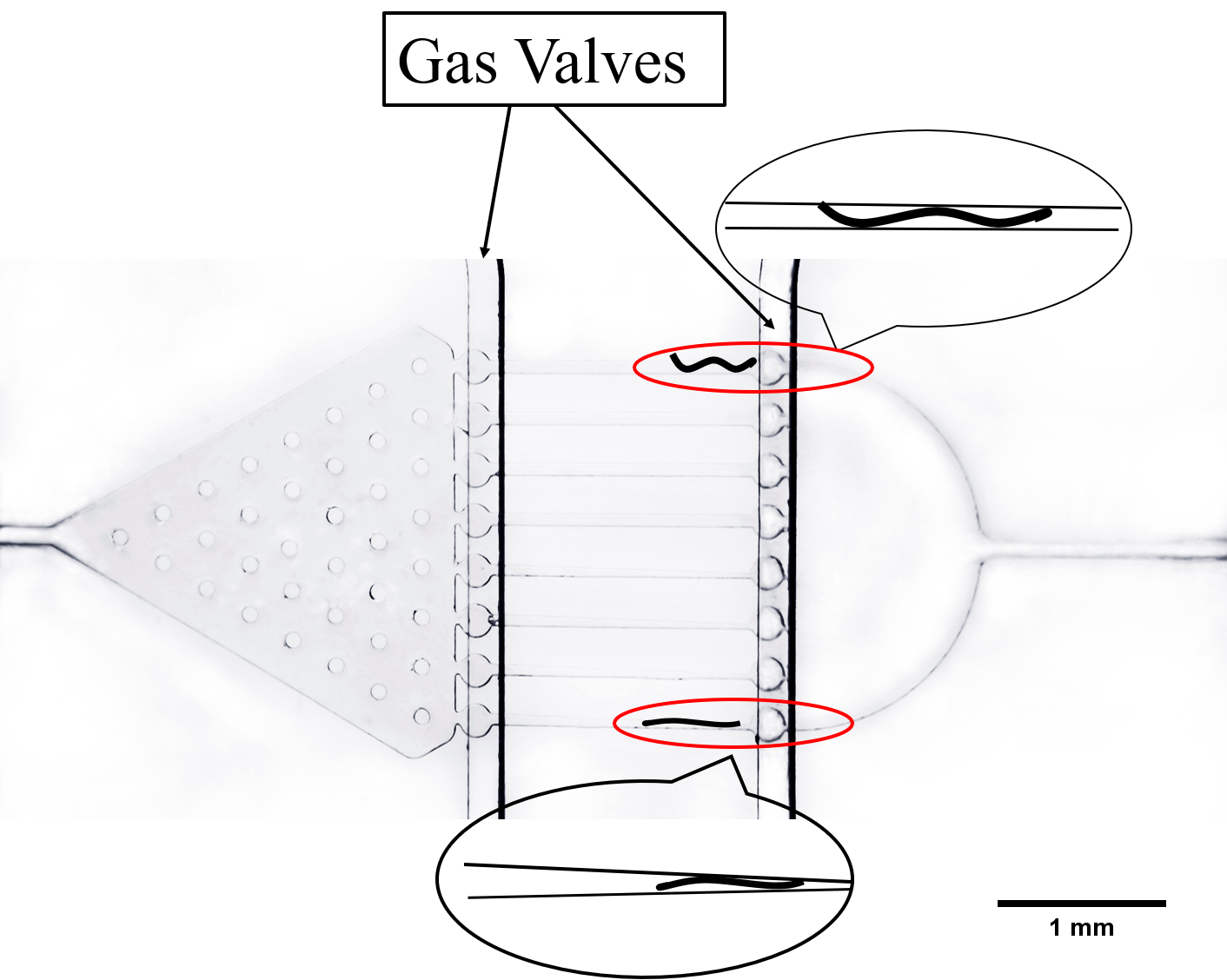
The second kind of channel is compressed and rectangular called parallel channel. Worms can be restricted by turning off gas valves in this compressing channel.
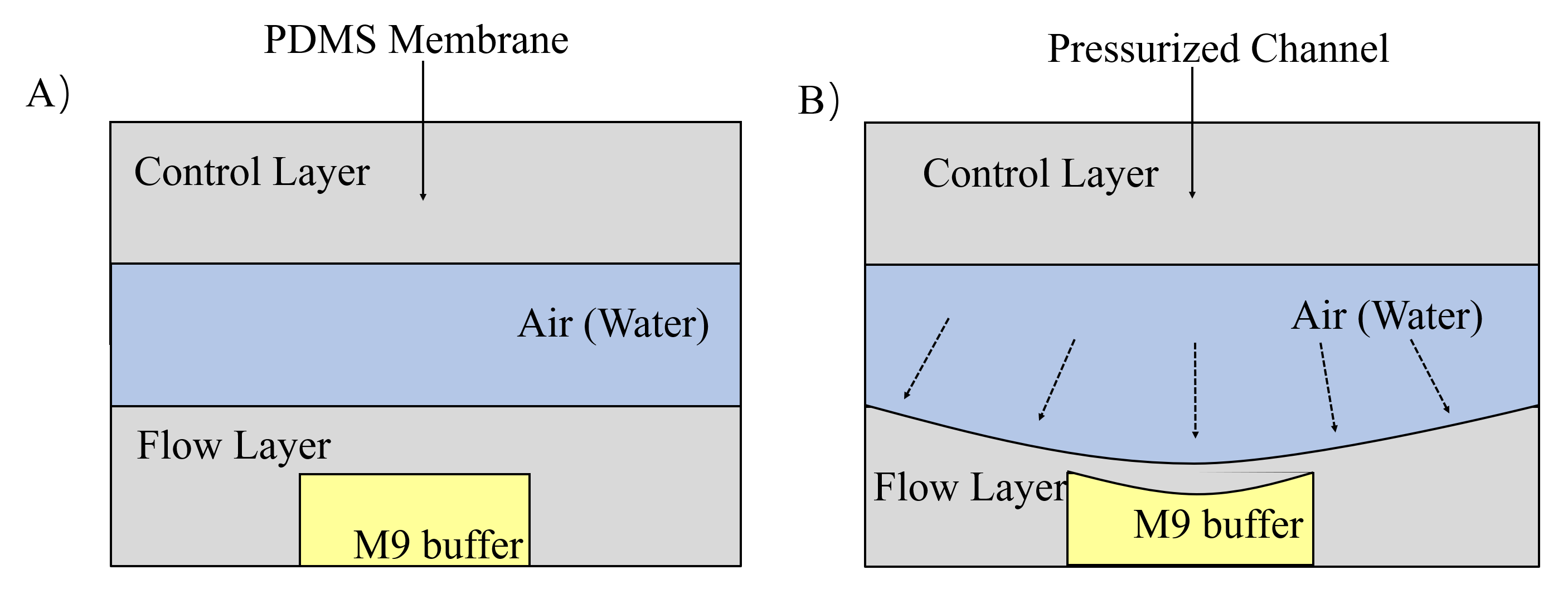
Both of these channels can restrict worms. But in case that they go away from channels, we designed gas valves to block their entry and exit by compressing PDMS (a flexibility and Easily deformed material) under the pressure made by water or air. The principle of gas valves is as below. (Fig. 9) [3]
The Fabricating And Using of Microfluidics
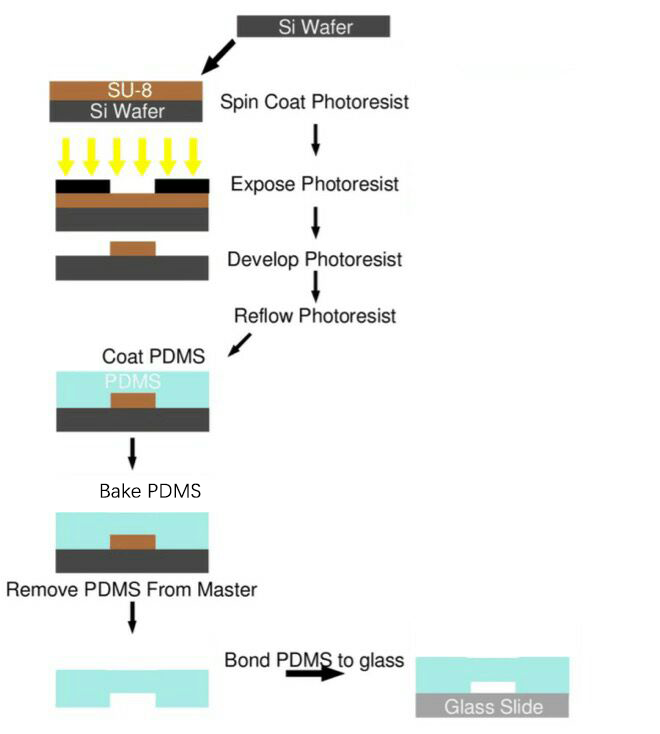
After designing these microfluidics, we need to fabricate them and utilize them. The microfluidics chips used in our project were all produced using this protocol by SUSTech_Shenzhen last year. (Fig. 10) Detailed Protocol
When injecting the flow and worms into microfluidics, We use pump to inject them and use microscope to observe the condition in chips. (Fig. 11)
The contents above are our all hardware parts in microfluidics. You can learn more details in results. Detailed Results
References
- ↑ Albrecht, D.R., and Bargmann, C.I. (2011). High-content behavioral analysis of Caenorhabditis elegans in precise spatiotemporal chemical environments. Nat. Methods 8, 599-605.
- ↑ Bean machine. (2017, October 5). In Wikipedia, The Free Encyclopedia. Retrieved 12:46, October 22, 2017, from https://en.wikipedia.org/w/index.php?title=Bean_machine&oldid=803992086
- ↑ Unger, M.A., Chou, H.P., Thorsen, T., Scherer, A., and Quake, S.R. (2000). Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 288, 113-116.