Contents
Overview
Campylobacter jejuni is the leading cause of food poisoning in the UK, and is commonly found on uncooked poultry. Our team aims to develop a functional biosensor to detect Campylobacter jejuni on contaminated surfaces. We aim to engineer E.coli to produce GFP in response to the presence of xylulose – a sugar found in the outer capsule of C. jejuni. This report focuses on utilization of the L-arabinose sensing AraC regulatory protein and its regulated PBAD promoter to construct one of the parts of the final detection system. The regulatory protein AraC binds to operator sequences in the PBAD promoter and activates transcription of downstream genes only when bound to its substrate sugar (L-arabinose). Here we performed saturation mutagenesis on the araC gene to construct AraC mutant library with four randomized ligand-binding pocket amino acid residues corresponding to positions 8, 24, 80 and 82. The AraC mutant library was subjected to fluorescence-based screening using a GFP reporter. Due to unavailability of xylulose we tested for mutants responsive to xylose, decanal or no effector. We isolated and sequenced 4 mutants which showed altered effector specificity. Our results demonstrate an approach that allows to engineer a regulatory protein to respond to an effector molecule of interest and represent steps towards developing a functional biosensor to detect C. jejuni on contaminated surfaces.
Background
Campylobacter jejuni is a bacterial pathogen found in animal gastrointestinal tract, and is mostly associated with chickens. It is responsible for a vast number of food poisoning cases around the world. Current detection systems for the pathogen are time-consuming, expensive and inaccessible for everyday users. Our team aims to develop a functional biosensor by engineering Escherichia coli to detect xylulose - a sugar naturally present in the outer capsule of Campylobacter jejuni [1]. The sugar can be easily released from the capsule by contact with acid. Our team aims to construct two alternative regulatory systems as potential components of the biosensor. One of them exploits the MtlR operon, naturally found in Pseudomonas fluorescens. MtlR protein activates transcription of downstream genes upon binding to xylulose. Parts of the operon can be transformed into E. coli and utilized in such a way that in contact with xylulose, MtlR protein activates transcription of a gene producing a detectable signal, such as fluorescence from GFP. However, this approach may have some disadvantages. Coming from a different organism, the MtlR protein may not function in E. coli cells as expected. Furthermore, the MtlR operon has not been previously exploited in biosensors and may not show sufficient specificity to xylulose. Hence, we aim to construct an alternative regulatory system which exploits the L-arabinose operon.
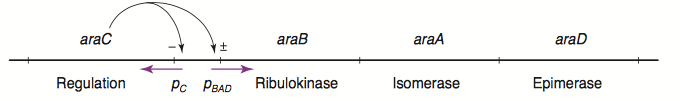
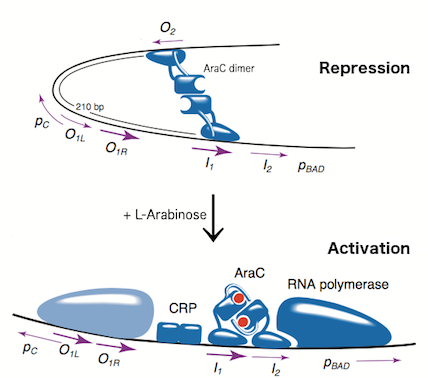
The L-arabinose operon is naturally found in Escherichia coli. The regulatory protein, AraC, acts as a dimer. As depicted in Fig. 1, the operon contains two regulatory cis elements: the PC promoter for the synthesis of AraC, and the PBAD promoter for synthesis of enzymes required for catabolism of L-arabinose. The mechanism of the operon regulation has been described by Schleif et al (2000) and is summarized in Fig. 2. The PBAD promoter contains 3 half sites that each bind to one subunit of AraC - O2, I1, I2. The O1 site is composed of O1L and O1R half sites, which bind both subunits. The O2 half site is within the araC coding region. In absence of L-arabinose the AraC dimer binds to operator half-sites O2 and I1. This causes DNA looping upstream of the PBAD promoter which represses transcription by excluding RNA polymerase from binding to PBAD or PC. Binding of L-arabinose causes a conformational change in the protein such that the DNA-binding domains of the dimer bind to adjacent I1 and I2 half-sites, giving access for RNA polymerase and cyclic AMP receptor protein (CRP) to bind PBAD. This results in transcriptional activation.
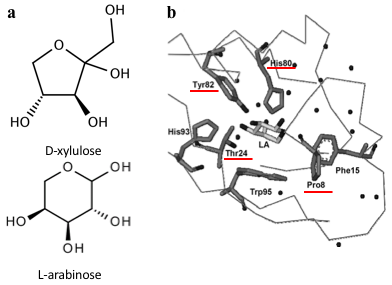
Several previous studies have shown that AraC protein can be engineered to activate transcription in response to non-native small molecules. Some of them include D-arabinose (enantiomer of L-arabinose)[4], mevalonate[5] and triacetic acid lactone[6]. Site-saturation mutagenesis of residues positioned within the ligand-binding pocket of AraC, coupled with fluorescence-based cell sorting, allowed the groups to isolate AraC variants with altered effector specificity. Based on these findings we aimed to use multiple site-saturation mutagenesis and fluorescence-based screening to generate mutant AraC responsive specifically to xylulose. Although xylulose comes in the form of two enantiomers, we chose to focus on D-xylulose, which is the isomer present in the capsule of C. jejuni (Gilbert et al, 2007). Both L-arabinose and D-xylulose are monosaccharides with five carbons, with molecular formulas C5H10O5. As seen in Fig. 3a, arabinose is an aldopentose with an aldehyde functional group at carbon position 1, while xylulose is a ketopentose, possessing a ketone group instead. The 3-dimensional structure of D-xylulose differs from that of L-arabinose, with the most pronounced difference being the 5-membered vs 6-membered heterocyclic ring in its structure. Fig. 3b represents key residues of AraC protein that play a role in ligand binding. Previous studies have shown that mutagenesis of amino acids in positions 8, 24, 80 and 82 is sufficient to change effector specificity[7].
Aims
- Aim 1
To split the AraC regulatory system into two plasmids: regulatory plasmid expressing AraC and AraC mutants and reporter plasmid expressing GFP under control of PBAD promoter.
- Aim 2
To perform multiple site-saturation mutagenesis on the AraC protein at residue positions 8, 24, 80 and 82.
- Aim 3
To isolate AraC variants with altered effector specificity using fluorescence-based screening.
Materials and Methods
Plasmid design
Plasmid construction was as follows.
Oligos containing parts R0011 (LacI-regulated promoter) upstream of B0032 (ribosome-binding site) were synthesised by Integrated DNA Technologies (IDT). Each strand had different, incomplete prefix and suffix sequences, such that when annealed the resulting dsDNA had BioBrick-compatible sticky ends for ligation into EcoRI and PstI digested BioBrick vectors. The resulting product was ligated into high copy number pSB1C3 vector digested with EcoRI and PstI. The ligation reaction was introduced into chemically competent E.coli strain DH5, and sequence from a chosen colony was verified.
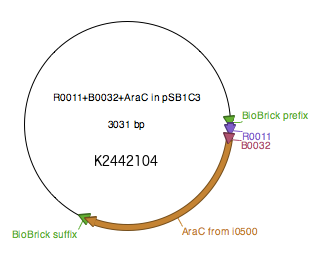
WT araC was amplified from BBa_I0500 using primers araC_BBPre_F and araC_BBSuf_R (see Table 1) to introduce BioBrick prefix and suffix to both ends of WT araC. The PCR product was subsequently digested with XbaI and PstI. pSB1C3-R0011-B0032 was digested with SpeI and PstI. The two digested parts were ligated to generate K2442104 plasmid with WT araC. Sequence was verified.
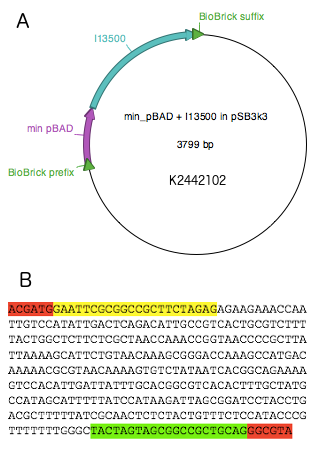
PBADmin was synthesised by Integrated DNA Technologies (IDT) as a G-block and digested with EcoRI and PstI. The cut product was then ligated into pSB3k3 vector digested with EcoRI and PstI. The ligation product was introduced into E.coli strain DH5 by chemical transformation and the sequence was verified. Part I3500 was then digested with enzymes XbaI and PstI and ligated into pSB3k3-PBADmin, digested with SpeI and PstI. This resulted in the final reporter plasmid K2442102, and the sequence was verified.
Library Construction
Primer sequences are listed in Table 1. araC gene was amplified from the BBa_I0500 part using primers araC_F and araC_R. The PCR product was then used in three PCR reactions with the following sets of primers: araC_Frag1_F and araC_Frag1_R; araC_Frag2_F and araC_Frag2_R; araC_Frag3_F and araC_Frag3_R. The PCR conditions were: 98°C for 30s, then 35 cycles of 98°C for 10s, 67.5°C (for F1 and F2) or 59.8°C (for F3) for 30s and 72°C for 1min, then final step of 72°C for 10 mins. The reactions resulted in 3 araC fragments (F1, F2 and F3). Primers araC_Frag1_F, araC_Frag1_R and araC_Frag2_R contain the degenerate sequences NNS at specific triplets so that fragments F1 and F2 contain saturation mutagenesis at codon positions 8, 24, 80 and 82. PCR products were gel purified and DNA concentration of each was measured with a NanoDropTM spectrophotometer. Equimolar aliquots (0.15pmol each) of adjacent fragments were combined (F1+F2 and F2+F3) and PCR-assembled without primers under the following conditions: 98°C for 30s, then 15 cycles of 98°C for 10s, 60°C for 1min and 72°C for 40s, then 72C for 10 mins. 15l of each reaction product was combined and PCR-assembled without primers under following conditions: 20 cycles of 98C for 30s and 72C for 40s. Finally primers araC_BBPre_F and araC_BBSuf_R were added and a final round of PCR was ran with the following programme: 98°C for 30s, then 30 cycles of 98°C for 10s, 60°C for 1 min and 72°C for 40 s, then 72°C for 10 min. QIAQuick PCR purification of the product was performed according to the Qiagen protocol.
The purified PCR product was then digested with XbaI and PstI and ligated into pSB1C3+R0011+B0032 digested with SpeI and PstI. Ligation reaction was ethanol-precipitated, and transformed into electrocompetent DH5 by electroporation. This resulted in the araC mutant library.
Colonies carrying the mutant library were washed off transformation plates with 1.5ml double distilled H2O, (ddH2O) then transferred to a 1.5ml tube. The cell pellet was centrifuged at room temperature, the supernatant was then discarded and pellet resuspended in 1.5ml ddH2O. This centrifugation-resuspension step was repeated 3 times to remove agar plate debris. Plasmid DNA was extracted using the Qiagen Plasmid MiniPrep Kit with 2 of each PB buffer and PE buffer wash steps to ensure maximum extract purity. Sequencing primer VF2 was then added and sample sent for sequencing.
Fluorescence-based screening
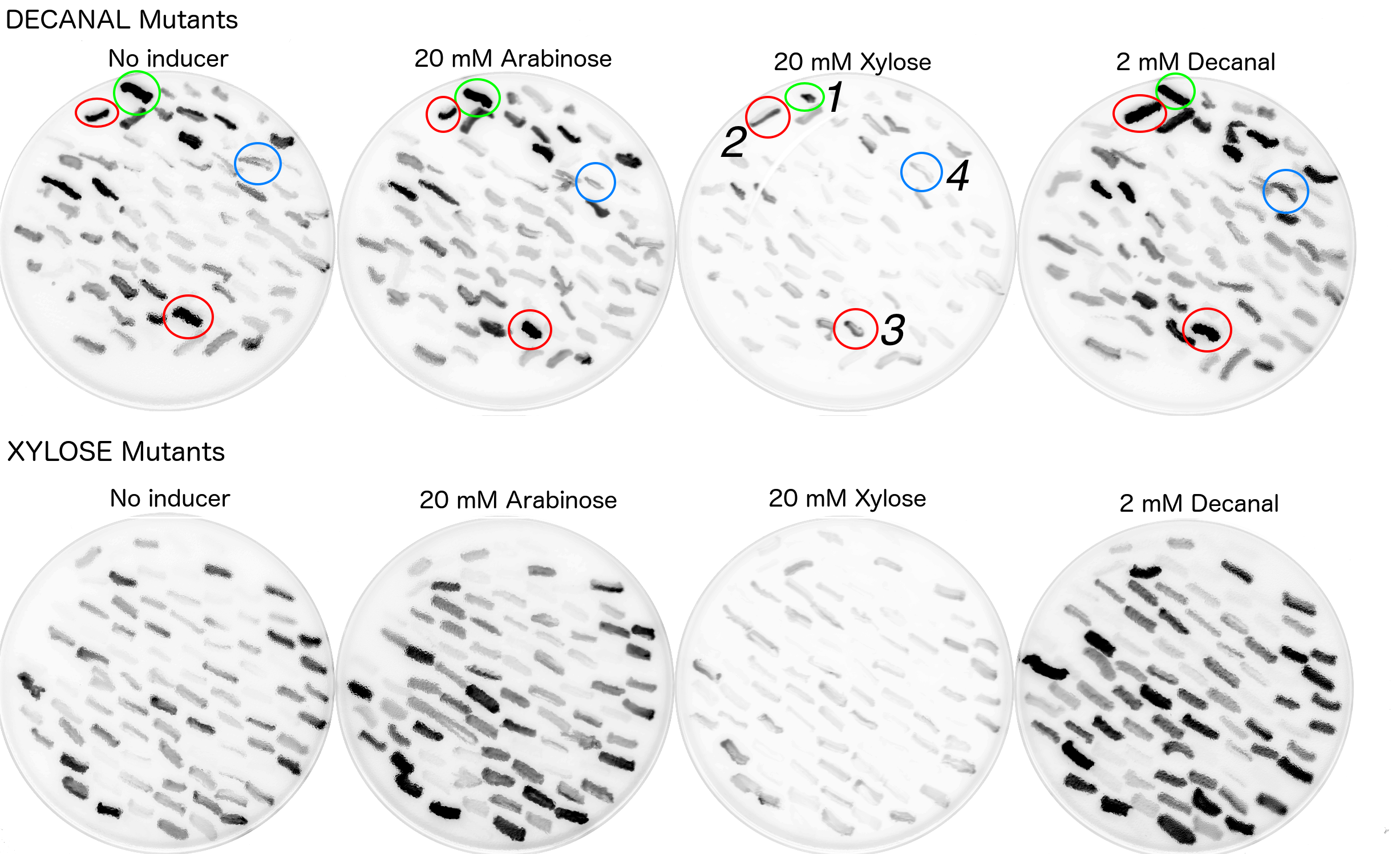
Plasmid DNA containing the araC mutant libraries were transformed into DS941 carrying the reporter plasmid K2442102 (in pSB3k3 vector). Transformants were plated on LB agar medium containing chloramphenicol plus kanamycin (to select for K2442102 and K2442104) plus one of the tested inducers: arabinose, xylose or decanal. Concentrations were 40mM for arabinose and xylose and 2mM for decanal. As a control test transformants were also plated on LB medium containing chloramphenicol and kanamycin only. Fluorescence images of the conditional transformation plates were obtained using a GE-Healthcare Typhoon FLA-9500 laser scanner. Excitation was recorded at 473nm, emission was recorded using 520-540nm filter. Colonies which exhibited fluorescence were observed as dark colonies on the scan (Fig. 8). 200 of those which appeared dark on xylose or decanal plates were re-streaked onto plates containing xylose, arabinose, decanal or no additive. Each colony of interest was picked and then immediately short-streaked onto each new condition plate using the same toothpick, into the same position using grids. This method of replica plating ensures the plates can later be aligned and short streaks of the same origin directly compared between the plates. After overnight incubation at 37°C, fluorescence scans of each plate were again obtained using the scan fluorescent scanning method.
Liquid culture fluorescence assay
Colony of interest was inoculated into L-broth containing chloramphenicol and kanamycin. Each liquid culture was grown overnight at 37°C, shaking at 225 rpm. The following day the culture was diluted 1:100 into fresh L-broth containing the above antibiotics plus one of the inducer conditions: 40mM xylose, 40mM arabinose, 2mM decanal. 200l of each culture to be tested was placed in a well of a clear-bases 6-well plate, then incubates at 37°C shaking at 300 rpm for 12 hours in a BMG Fluorostar Omega fluorescence plate reader. Fluorescence of each culture was measured at 1 hour time intervals during the culture experiment, using excitation wavelength 485nm and emission wavelength 530nm. Cell growth was simultaneously tracked by measuring optical density at 600 nm.
Results
Regulatory plasmid K2442104 contains wild type or mutant araC gene under control of LacI-regulated promoter R0011 linked to ribosome binding site B0032. This is to enhance control over production of the AraC, so that the growth of bacteria is not affected by overproduction of the protein. Production of AraC can be stimulated by addition of Isopropyl β-D-1-thiogalactopyranoside (IPTG) to the media. IPTG mimics allolactose and prevents LacI from binding to its operator sequences in the DNA, switching on the R0011 promoter. The construct was ligated into pSB1C3 plasmid backbone (Fig. 4).
The reporter plasmid K2442102 contains GFP gene downstream of minimal PBAD promoter (PBADmin) (Fig. 5A). Minimal PBAD contains minimal DNA sequence from the native PBAD that is sufficient to retain its function (Figure 5B). The promoter retains all the operator sites O1, O2, I1 and I2 required for binding of AraC. As these sites lie within the PC promoter and overlap with AraC coding sequence, the minimal PBAD has the araC start codon ATG mutated to AGT to prevent the protein from being made. PBADmin was created and tested with the help of Steven Kane, member of Colloms laboratory. To enable incorporation of PBADmin into a BioBrick-compatible plasmid, we added BioBrick prefix and suffix sequences to either site of the PBADmin sequence (Fig. 5B). Part K2442102 was expected to work as follows. When AraC is activated by its binding substrate, it binds to I1 and I2 half sites within PBADmin and drives expression of GFP, causing the host cell to produce green fluorescence. For the purposes of testing, the construct was ligated into pSB3k3 plasmid backbone. The plasmid has a different origin of replication from that of pSB1C3, therefore both plasmids can co-exist in the same E. coli cell.
Discussion
Outlook
- 1
- 2
- 3
References
- ↑ Gilbert, M., Mandrell, R., Parker, C., Li, J., and Vinogradov, E. (2007). Structural Analysis of the Capsular Polysaccharide from Campylobacter jejuni RM1221. Chembiochem 8, 625-631
- ↑ Schleif, R. (2000). Regulation of the L-arabinose operon of Escherichia coli. Trends In Genetics 16, 559-565..
- ↑ Schleif, R. (2000). Regulation of the L-arabinose operon of Escherichia coli. Trends In Genetics 16, 559-565..
- ↑ Tang, S., Fazelinia, H., and Cirino, P. (2008). AraC Regulatory Protein Mutants with Altered Effector Specificity. Journal Of The American Chemical Society 130, 5267-5271.
- ↑ Tang, S., and Cirino, P. (2010). Design and Application of a Mevalonate-Responsive Regulatory Protein. Angewandte Chemie 123, 1116-1118.
- ↑ Frei, C., Wang, Z., Qian, S., Deutsch, S., Sutter, M., and Cirino, P. (2016). Analysis of amino acid substitutions in AraC variants that respond to triacetic acid lactone. Protein Science 25, 804-814.
- ↑ Tang, S., Fazelinia, H., and Cirino, P. (2008). AraC Regulatory Protein Mutants with Altered Effector Specificity. Journal Of The American Chemical Society 130, 5267-5271.