Contents
Challenge
One of the major experimental limitations of our design was the reliance on using genetically modified E. coli cells as the method of detection due to the relative difficulty and inefficiency of storing them such that the cells remain viable. As described, a polyacrylamide hydrogel matrix was used to immobilize the E. coli cells which, to maintain cell viability, had to be stored at refrigeration temperature. Even then, in our tests, the cells lost viability after a couple of days. This problem led to the necessity of finding a different organism that would be better suited to shuttling our genetic construct for a longer period of time under a wider range of conditions.
Solution
One of our advisors, James Provan, suggested using the bacteria Bacillus subtilis due to its ability to form endospores which are extremely resilient under a vast range of conditions such as high temperatures and lack of nutrients (Tan and Ramamurthi, 2013)[1]. As the decision to use an alternative organism to E.coli was made approximately half-way through our project, there was not sufficient time to completely redesign our genetic construct such that it could be expressed in Bacillus subtilis. Because of this, a new experiment had to be thought off to show that the bacteria could be used for our purposes. One of our supervisors, Sean Colloms, got in contact with a researcher from Newcastle University, Richard Daniel, who kindly sent us a sample of Bacillus subtilis cells that had been genetically modified such that they became blue-green when exposed to the sugar X-Gal. The proposed experiment involved sporulating these cells and then reanimating them on plates containing X-gal to observe if the cells maintained their genetic modification after sporulation and be able to show a colour change, much like we were aiming to do with our genetic construct.
Preliminary Results
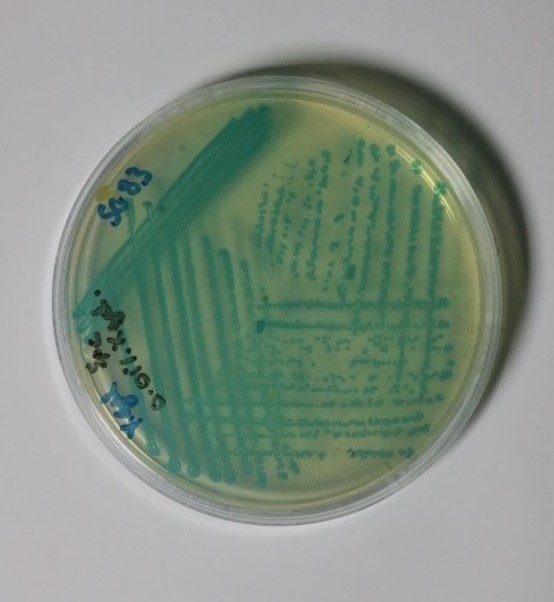
Upon receipt of the Bacillus subtilis containing plate from Newcastle University (shown in figure 1 below), various preliminary tests were performed to find the optimum conditions for the growth of the bacteria.
- The first test performed involved picking two colonies from the original plate and streaking them on plates, one containing solely LB-agar medium and one containing a LB-agar and X-gal (final concentration of 40μg/ml in agar) medium. The plates were then incubated overnight at 37°C.
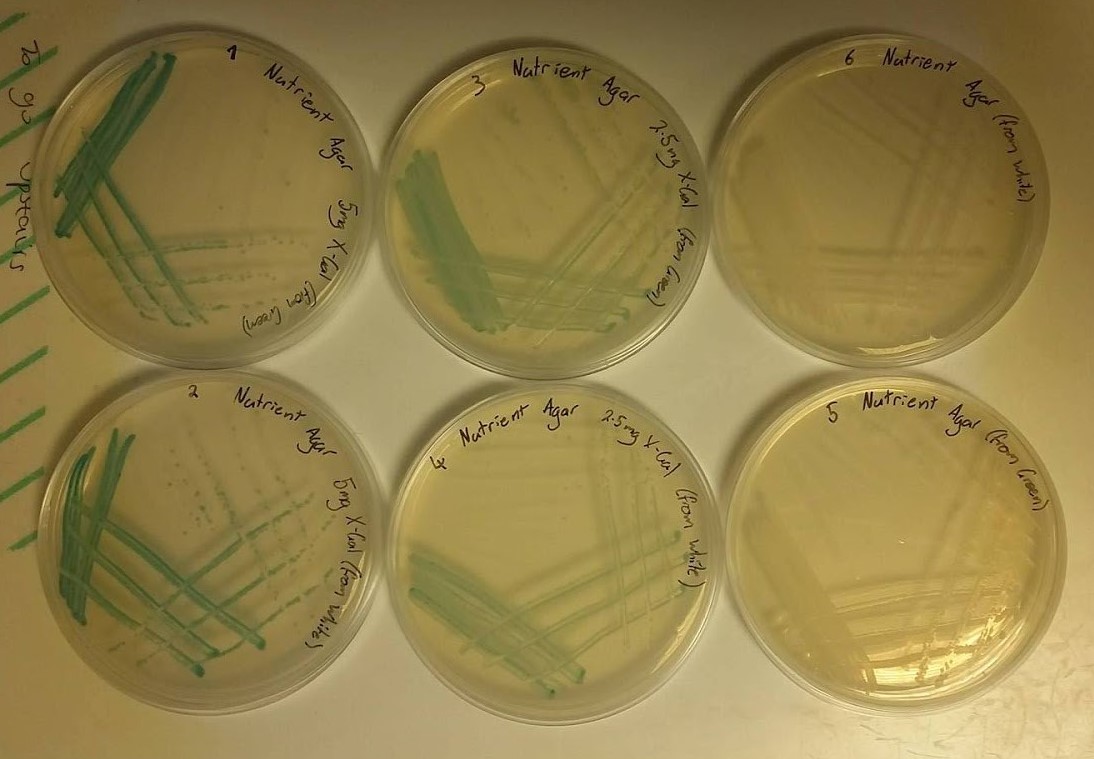
Unfortunately, no photographs of the plates were taken at the time, however, both plates showed growth of white colonies. This was expected in the plate containing no X-gal however, it was expected that the other plate should have showed growth of blue-green colonies indicating that the concentration of X-gal was not sufficient enough to make the colonies change colour. Additionally, the morphology of all of the colonies did not show healthy growth, noted by “spikey” edges of each colony, which suggested that LB-agar was not the correct medium to grow Bacillus subtilis on. Because of this it was decided that in future tests that nutrient agar would be used in addition to a higher concentration of X-gal. - To confirm that the correct conditions for the growth and colour change of Bacillus subtilis had been met, a second test was performed. Six plates were set up as follows:
- 25ml nutrient agar, 50μl of X-gal stock solution (100mg/ml) giving a final concentration of X-gal in agar of 200μg/ml and streaked with a colony from the original plate (blue-green) that was sent from Newcastle University.
- 25ml nutrient agar, 50μl of X-gal stock solution (100mg/ml) giving a final concentration of X-gal in agar of 200μg/ml and streaked with a colony from the first plate of the first test (white).
- 25ml nutrient agar, 25μl of X-gal stock solution (100mg/ml) giving a final concentration of X-gal in agar of 100μg/ml and streaked with a colony from the original plate (blue-green) that was sent from Newcastle University.
- 25ml nutrient agar, 25μl of X-gal stock solution (100mg/ml) giving a final concentration of X-gal in agar of 100μg/ml and streaked with a colony from the first plate of the first test (white).
- 25ml nutrient agar, no X-gal and streaked with a colony from the original plate (blue-green) that was sent from Newcastle University.
- 25ml nutrient agar, no X-gal and streaked with a colony from the first plate of the first test (white).
- These plates were incubated overnight and the growth was observed in the morning as shown in the following image (figure 2).
- As can be observed from the image, all of the colonies on each plate exhibit healthy morphologies, something that did not happen in the first test. This can be attributed to the use of nutrient agar rather than LB-agar. Plates 1, 2, 3 and 4 all show growth of colonies of the blue-green colour associated with X-gal screening tests with the darker colour shown by plates 1 and 2 being a result of the higher concentration of X-gal used. Plates 5 and 6 were expected to show growth of white colonies as no X-gal was present in the nutrient agar medium. These results informed us that in future tests, nutrient agar and an X-gal concentration of 200μg/ml should be used.
Sporulation of Bacillus subtilis
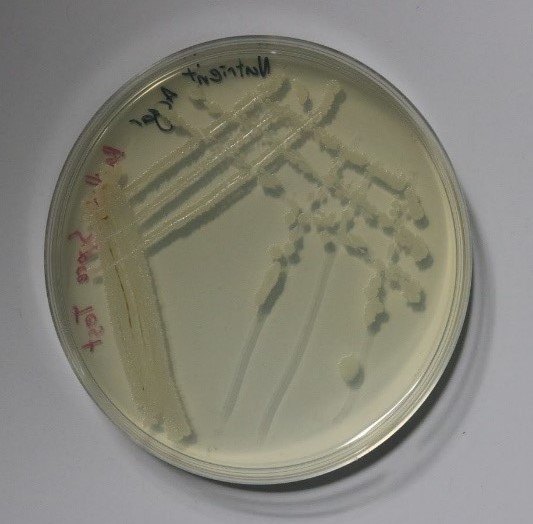
As the optimum conditions for growing Bacillus subtilis had been found, the main experiment proposed could now begin. The first stage of this experiment involved initiating sporulation of the cells such that they produced endospores. This was achieved by following and slightly editing the protocol developed by the collaboration between the Bonn and Freiburg iGEM teams from 2016 (iGEM Bonn 2016 and iGEM Freiburg 2016, 2016)[2]. Once the sporulation of the cells had been successful, the spores were stored in a refrigerator for two days where the cold temperature would be detrimental to normal Bacillus subtilis cell growth. To ensure that there were no live, unsporulated cells remaining, the eppendorf tube containing the spores was placed into a dry heat block at 90°C for 10 minutes which would be high enough to kill any normal cells. To reanimate the spores and to observe if they preserved the mutation that allowed the cells to change colour after sporulation, the spores were streaked on two plates, one containing nutrient agar and 200μg/ml of X-gal and one containing only nutrient agar as a control. The plates were incubated overnight and the results are shown in the figures 3 and 4 below.
As can be seen in these images, the growth on the plate that contained X-gal turned blue-green and the growth on the plate without X-gal stayed white as expected. This proves that throughout the sporulation process, the Bacillus subtilis cells retained their mutation that allowed for a colour change to occur.
Conclusion
As such, we can conclude that this bacterium would be ideal for use in our design should we have the chance to work on it for a longer length of time. The Calgary 2014 iGEM team worked on a Bacillus subtilis based biosensor which could provide further knowledge should a team want to explore this avenue in the future.
References
- ↑ Tan, I. and Ramamurthi, K. (2013). Spore formation in Bacillus subtilis. Environmental Microbiology Reports, 6(3), pp.212-225.
- ↑ iGEM Bonn 2016 and iGEM Freiburg 2016 (2016). How To Bacillus subtilis. [pdf] p.9. Available at: https://static.igem.org/mediawiki/2016/8/8c/T--Freiburg--BacillussubtilisManueliGEMFreiburgandBonn2016new3.pdf [Accessed 7 Aug. 2017].