Contents
Overview
As live E. coli cells were to be used in our design, a way of immobilizing them had to be found so that they would not be able to escape into the environment but still be able to interact with the target molecule, xylulose, whilst still remaining viable. A number of immobilization techniques were assessed but, ultimately, hydrogel encapsulation was chosen due to the ease of production and for its ability to allow the free diffusion of water soluble molecules, such as xylulose. Since polyacrylamide hydrogels are used in standard biology experiments, it was chosen as the matrix in the first instance. We also developed a method based on wax-bounded filter paper strips to immobilise the gel as discussed below.
Pore Size
Initial research into the production of polyacrylamide hydrogels revealed that the intrinsic properties of the gel, such as pore size, are dependant on the composition of the gel itself (Nationaldiagnostics.com, n.d.)[1]. Polyacrylamide gels are composed of five components; acrylamide in aqueous solution, the crosslinker bisacrylamide, two reaction initiators, ammonium persulfate (APS) and tetramethylethylenediamine (TEMED), and a buffer, such as phosphate-buffered saline (PBS). The total percentage concentration of acrylamide in the gel directly controls the pore size of the gel with a higher percentage resulting in smaller pores. The weight ratio between the acrylamide and crosslinker also has an effect on the pore size however the relationship is more complex (Nationaldiagnostics.com, n.d.)[1]. It was hypothesised that in order to better facilitate the diffusion of xylulose through the gel, that a larger pore size should be used. It was for this reason that, in preliminary tests to gain experience of preparing these gels, a 5% acrylamide gel was prepared as per the protocols section.
E. coli Viability in the Gels
In a bid to assess the viability of E. coli cells in the polyacrylamide gel, a test was performed whereby two gels were made up, as per the protocols section but before the polymerisation initiators were added, 2ml of overnight culture containing E. coli cells was added to each and thoroughly mixed. One gel was kept at room temperature and the other was kept at refrigeration temperature. A toothpick was then used to prick the gel and obtain a sample which was then streaked on a plate containing LB-agar. The gels were pricked every hour for a total of 4 hours. The plates were then incubated at 37°C overnight. The largest growth from the gel that was kept at room temperature was at the zero minute time interval with decreasing growth at each subsequent interval. Comparing this to the plate streaked from the gel that was kept at refrigeration temperature, where there was large growth at all intervals, shows that, in order for the cells to remain viable, the hydrogels need to be kept in the refrigerator. This test was repeated on the same gels, kept in the same conditions, for an extra day to increase the sampling period of the experiment however, on the following day, neither plate showed any signs of growth.
There were a few limitations with this experiment, such as, that when pricking the gel with a toothpick, there is no guarantee that any bacterial cells will adhere to the stick and that when spreading on the plate, the cells may remain within the sections of gel picked up by the stick and not actually be spread onto the plate. All of these limitations reduced the reliability of the results gained from this experiment.
Filter-Paper Strips
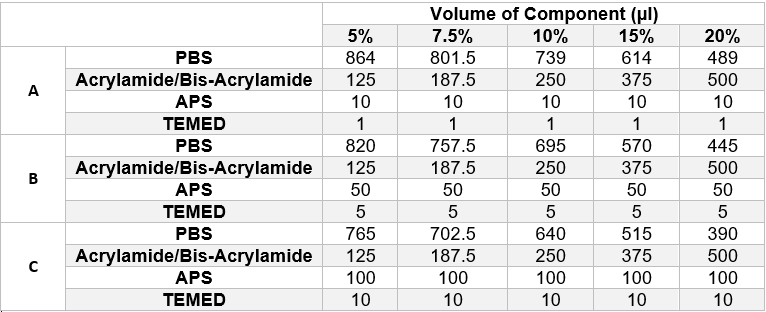
We designed filter paper strips which were surrounded by wax ink (Carrilho, Martinez and Whitesides, 2009)[2] which acted as a hydrophobic barrier and contained the gel to the area within the boundary. The wax boundary was designed so that the inner boundary would have the same dimensions as those of the detection chamber so that the strip could be trimmed down and placed into the device. The underside of the strip was completely covered in wax ink to prevent the liquid from passing through the strip. To find the ideal combination of reactants to polymerise the gel quickly enough for it to set on the paper, an experiment was set up that would sample a wide range of different gels. Fifteen paper strips were laid out in five different petri dishes. They were then loaded with one of the following combinations of reactants, as shown in table 1 below, and left to polymerise at room temperature once the petri dishes had been sealed. The gels were prepared in the same manner as described in the protocols section.
After approximately 30 minutes, the paper strips were inspected to observe if any of the gel combinations had successfully polymerised on the strips. The results from this test are shown in the image below (figure 1) however it is quite difficult to determine how successful each gel was due to image quality. The image does, however, show the design of the wax ink coated paper strips.
None of the gels in row A of the table above (APS volume = 10μl, TEMED volume = 1μl) had polymerised after 30 minutes however all other gels had polymerised to varying degrees. The three most successful gels, and the combinations that would be used in future tests, were as follows:
- 7.5% Acrylamide Gel, 100μl APS, 10μl TEMED
- 10% Acrylamide Gel, 100μl APS, 10μl TEMED
- 15% Acrylamide Gel, 50μl APS, 5μl TEMED
Out of these three gels, the 7.5% gel was selected as the best option due to larger pore sizes. It should be noted that the same stock acrylamide solution (30% Acrylamide/Bis-acrylamide Solution (37.5:1 weight ratio)) as used in the preliminary tests was used for this experiment and that the different percentages of acrylamide in each gel was achieved by calculating the correct volume of stock solution to add.
Future Work
Due to time restraints, there was no time to perform another cell viability test for the newly selected optimum gel. Neither was there time to perform an experiment where the cells in the gel physically detected the target molecule, xylulose, which would have proved if the idea of encapsulating live E. coli cells in a polyacrylamide matrix would work for use in our biosensor.
References
- ↑ 1.0 1.1 Nationaldiagnostics.com. (n.d.). The Polyacrylamide Matrix | National Diagnostics. [online] Available at: https://nationaldiagnostics.com/electrophoresis/article/polyacrylamide-matrix [Accessed 17 Jul. 2017].
- ↑ Carrilho, E., Martinez, A. and Whitesides, G. (2009). Understanding Wax Printing: A Simple Micropatterning Process for Paper-Based Microfluidics. Analytical Chemistry, 81(16), pp.7091-7095.